
- 514 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Materials for Energy
About this book
Materials for Energy offers a comprehensive overview of the latest developments in materials for efficient and sustainable energy applications, including energy conversion, storage, and smart applications.
- Discusses a wide range of material types, such as nanomaterials, carbonaceous electrocatalysts and electrolytes, thin films, phase change materials, 2D energy materials, triboelectric materials, and membrane materials
- Describes applications that include flexible energy storage devices, sensors, energy storage batteries, fuel and solar cells, photocatalytic wastewater treatment, and more
- Highlights current developments in energy conversion, storage, and applications from a materials angle
Aimed at researchers, engineers, and technologists working to solve alternative energy issues, this work illustrates the state of the art and latest technologies in this important field.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
Physical SciencesSubtopic
Energy1 Halide Perovskite Photovoltaics
Guifang Han
Shandong University
Sam Zhang
Southwest University
CONTENTS
1.1 Solar Energy and Photovoltaics
1.2 Halide Perovskite Materials
1.2.1 Structure of Halide Perovskite Materials
1.2.2 Optical Property and Bandgap Tunability of Halide Perovskite Materials
1.2.3 Optoelectronic Property of Halide Perovskite Materials
1.3 Stability of Halide Perovskites
1.3.1 Intrinsic Thermal Stability of Halide Perovskite Materials
1.3.2 Phase Stability of FAPbI3 Perovskite
1.3.3 Phase Stability of All-Inorganic CsPbI3 Perovskite
1.4 Summary
References
1.1 Solar Energy and PhotovoltaicS
The sun not only gives us light and happy mood, but is also a natural power source for our Earth. It drives the circulation of global wind and ocean currents, the evaporation and condensation of water cycle that creates rivers and lakes, and the biological cycle of photosynthesis that causes the diversity of nature and life (Lewis and Crabtree 2005). It provides us clean and abundant energy. The energy from sunlight to our Earth in 1 hour is more than the total energy consumption by humans in an entire year (Lewis and Crabtree 2005, Lewis and Nocera 2006, Cook et al. 2010). The energy released by the earthquake in San Francisco 1906 with a magnitude of 7.8 is equal to the amount of energy the sun delivers to the Earth in 1 second (Crabtree and Lewis 2007). Solar energy is the largest resource among various renewable energy sources by far. Solar energy can be converted to electricity through photovoltaic cells, fuel through natural or artificial photosynthesis, and thermal energy by heat engines or other techniques (Crabtree and Lewis 2007). Here in this chapter, we focus on how to convert solar energy into electricity through photovoltaic cells, emphasizing the principle of photovoltaics, perovskite materials, and the structure and properties of these materials.
Photovoltaic or solar cell is a device that absorbs light and converts it into electricity. Normally, for a semiconductor with a bandgap of Eg, it absorbs light with energy (hν) higher than its bandgap, and an electron is excited into the conduction band leaving a hole behind in the valence band (Figure 1.1a). If the excited electron could be collected and passed through an outer circuit, electricity is “generated”. Figure 1.1b depicts a typical current–voltage (I–V) curve of a solar cell. The open circuit voltage (Voc) is the maximum voltage that a device could obtain. The short circuit current (Jsc) is the maximum current that a device could achieve. The power conversion efficiency (η) of one solar cell is the ratio of the output electricity to the input energy of sunlight. In practice, the efficiency η is determined as the ratio of the maximum power output, Pmax, generated by the solar cell to the power input, Pin, based on the measurement of I–V curve: (Würfel 2007)
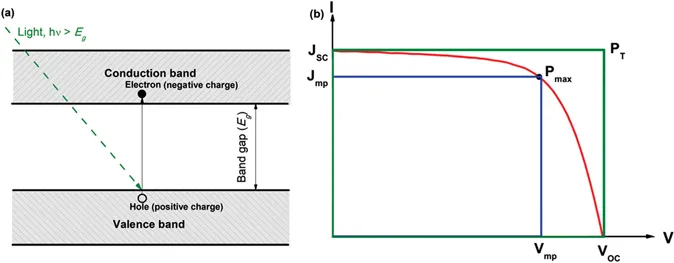
FIGURE 1.1 (a) Illustration of band structure and photoelectric effect in a bulk semiconductor and (b) a typical current–voltage (I–V) curve of a solar cell device for efficiency calculation (Han et al. 2017). (Reprinted with permission from Elsevier.)
where Jmp and Vmp are the current density and voltage at the maximum power point (Figure 1.1b). To simplify the calculation and relate the efficiency with practically measurable parameters, fill factor (FF) is introduced, which is defined as the ratio of the areas of two rectangles determined by Jmp and Vmp (blue in Figure 1.1b) and by Voc and Jsc (green in Figure 1.1b), respectively. Accordingly, the three parameters of Voc, Jsc, and FF combine to determine the efficiency of a device as shown in Eq. (1.1). The input energy of sunlight (Pin) is 100 (mW cm−2) based on one sun condition as a standard level for comparison of efficiency of devices fabricated in different labs and geographic conditions.
Before going into details of how to improve the efficiency of solar cell, we first look at the spectrum and energy distribution of standard sunlight shown in Figure 1.2 (based on solar cell application). Sunlight is actually electromagnetic radiation. According to the wavelength from lower to higher, people divide the spectrum of sunlight into three regions: ultraviolet (UV, wavelength of 300–400 nm), visible (VL, wavelength of 400–700 nm), and infrared (IR, wavelength of 700–4,000 nm) as presented in Figure 1.2a. The total power of the solar spectrum is integrated to 100 mW cm−2 at AM 1.5 G, which is usually named as standard one sun condition. Currently, most of solar cells utilize UV and visible regions of the solar spectrum. Near infrared (NIR, wavelength of 700–1,400 nm) is also used by some types of solar cells. The rest of the energy in the long-wavelength region is lost as heat. The energy level of photons, as shown in Figure 1.2b, can be calculated by the Planck–Einstein relation (French and Taylor 1978):
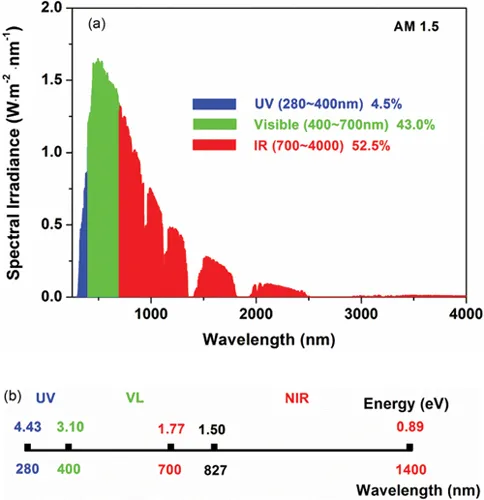
FIGURE 1.2 (a) Energy distribution of solar spectrum and (b) the corresponding energy level and wavelength. (Drawn based on the data of AM 1.5G according to ASTM G173) (Han et al. 2017). (Reprinted with permission from Elsevier.)
where h is the Planck constant, ν is the light frequency, c is the speed of light, and λ is the wavelength of incident light. This relation accounts for the quantum nature of light. The short-wavelength UV light, i.e. highest energy, only occupies <5% of the total solar energy. While the long-wavelength, low-energy NIR accounts for 52.5% of the solar energy. The visible light located in the middle covers around 43% of the solar energy.
The Eg of the absorber used limits the maximum value of Voc. Therefore, the higher the Eg, the higher the possible value of output Voc. The Jsc is a product of light harvesting efficiency, charge separation efficiency, and charge collection efficiency. The light harvesting efficiency is dependent on the absorbance of a semiconductor. The more photons are absorbed, the more efficient the light harvesting is. In principle, a semiconductor can only absorb a photon whose energy is higher than its Eg. However, photons bearing much higher energy than Eg can excite electrons to energy levels above conduction band minimum (CBM), and subsequently electrons rapidly relax to the CBM by releasing the extra energy as heat. To harvest more photons, the Eg should be as low as possible. Consequently, there exists an optimal bandgap energy for photovoltaic application in consideration of the spectrum losses. Shockley and Queisser calculated a theoretical conversion efficiency of around 31% for single junction solar cells (Shockley and Queisser 1961). M. Green proposed a simple empirical relation to estimate the minimal value of the reverse saturation current density. As such, the optimized Eg should be around 1.5 eV, which agrees well with the experimental data (Shah 2010, Green 1982).
Halide perovskite solar cell is a new type of thin film solar cell. Since its first report in 2009, the efficiency in 2019 already reached to more than 25%, which is the highest among all polycrystalline thin film solar cells. The low-cost solution process and feasibility of bandgap tuning make this material a promising candidate for novel thin film photovoltaics. Section 1.2 elaborates the feature structure and optical and optoelectronic properties of these materials. Section 1.3 explores the stability of halide perovskites including intrinsic thermal stability and strategies developed to stabilize these perovskite structures. A summary follows in Section 1.4.
1.2 Halide Perovskite Materials
The term “perovskite” originates from the mineral structure of CaTiO3, named after a Russian geologist Count Lev Aleksevich von Perovski (Tanaka and Misono 2001). Nowadays, perovskite refers to one big group of compounds, ABO3, which has a crystal structure similar to that of CaTiO3. It is one of the very important and most frequently encountered structures in solid-state inorganic compounds (Roy and Muller 1974). Perovskite covers most of the metallic ions in the periodic table with a significant number of anions. Perovskite is a very important crystal structure due to its diverse physical and chemical properties, such as ferroelectricity, piezoelectricity, and magnetoresistance, thermistor, electro-optical modulator, battery materials, and so on, playing an important role in the modern electronic industry (Li, Soh, and Wu 2004).
In an ideal cubic ABO3 perovskite structure, the A-site is a bigger cation (such as Ca2+, Sr2+, Ba2+), B-site is a smaller cation (such as Ti4+, Zr 4+), in which A is located at the corner of the cubic, B occupies the center of the cubic, and oxygen (O) fills the face center position. When the O is replaced by halogen (such as F, Br, Cl, I), A and B sites should be monovalent and divalent cations in order to keep neutral. This is halide perovskite.
This section discusses the structure...
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Editor
- Contributors
- Chapter 1 Halide Perovskite Photovoltaics
- Chapter 2 Carbon Nanomaterials for Flexible Energy Storage Devices
- Chapter 3 Triboelectric Materials for Nanoenergy
- Chapter 4 III-N Ultraviolet Light Emitters for Energy-Saving Applications
- Chapter 5 In-situ Growth of Spherical Graphene Films on Cemented Carbide for Spatial Sensor Matrix
- Chapter 6 Membrane Materials for Vanadium Redox Flow Battery
- Chapter 7 Thin-Film Solid Oxide Fuel Cells
- Chapter 8 In-Situ Mechanistic Study of Two-Dimensional Energy Materials by Well-Defined Electrochemical On-Chip Approach
- Chapter 9 Phase Change Materials for Thermal Energy Storage
- Chapter 10 Strategies for Performance Improvement of Organic Solar Cells
- Chapter 11 Surface Passivation Materials for High-Efficiency Silicon Solar Cells
- Chapter 12 Organic Solar Cell
- Chapter 13 High-Performance Electrolytes for Batteries
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Materials for Energy by Sam Zhang in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Energy. We have over one million books available in our catalogue for you to explore.