
This is a test
- 872 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Toxinology
Book details
Book preview
Table of contents
Citations
About This Book
Organized primarily around the mechanisms of action of the toxins at the biochemical, physiological and pathological level, rather than by source, the handbook covers most toxins which have been clearly identified and characterized, but emphasizes toxins that are more important by virtue of the sign
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Handbook of Toxinology by W. T. Shier, W. T. Shier in PDF and/or ePUB format, as well as other popular books in Medicine & Toxicology. We have over one million books available in our catalogue for you to explore.
Information
1
Cytolytic Toxins
ALAN L. HARVEY
Department of Physiology and Pharmacology, University of Strathclyde, Glasgow, United Kingdom
- INTRODUCTION
- BIOLOGY OF THE TARGET ORGAN
- CYTOLYTIC TOXINS IN GENERAL
- Pore-forming Toxins
- Cytolytic Enzymes
- Detergent-like Toxins
- Unknown Mechanisms
- SPECIFIC TOXINS
- Pore-forming Toxins
- Thiol-activated Cytolytic Toxins
- Staphylococcus aureus α-toxin
- Streptolysin S
- Delta-toxin (δ-toxin) of Staphylococcus aureus
- Hemolysins from Aeromonas hydrophila
- Cytolysin from Pseudomonas aeruginosa
- Hemolysin from Escherichia coli
- Channel-forming antibiotics
- Channel-forming toxins from sea anemones
- Channel-forming toxins from jellyfish
- Palytoxins
- Channel-forming toxins from bees, wasps, and hornets
- Pardaxins
- Cytolytic Enzymes
- Phospholipase A
- Phospholipase C
- Phospholipase D
- Detergent-like Toxins
- Cytolytic components from basidomycetes
- Saponins
- Prymnesin
- Toxins of Unknown Mechanisms
- Cardiotoxins from cobra venoms
- Cytotoxins from Cerebratulus lacteus
- Vibrio cytolytic toxins
- Other toxins of bacterial origin
- CONCLUSIONS
- REFERENCES
I. Introduction
Cytolytic toxins act directly on cell membranes. They disturb the normal physiology of the target cell and ultimately kill the cell. Cytolytic toxins are not a single group of related chemicals produced by one class of organism; rather, they are very heterogeneous in their chemical structures and they can be obtained from plant, microbial and animal sources. Consequently, cytolytic toxins do not share a common mechanism of action: there are several ways for exogenous toxins to interfere with the normal permeability barrier formed by the cell membrane. This is reflected in the wide range of potencies displayed by cytolytic toxins (TABLE 1).
Toxin | Molecular weight | LD50 in mice | |
(daltons) | (μg/kg) | (pmoles/kg) | |
Palytoxin (coelenterate) | 2,700 | 0.15 | 0.06 |
δ-Toxin (Clostridium perfingens) | 42,000 | 6 | 0.14 |
Streptolysin O (Streptococci spp.) | 67,000 | 10 | 0.15 |
β-Haemolysin (Aeromonas hydrophila) | 50,000 | 20 | 0.4 |
Cytolysin (Pseudomonas aeruginosa) | 23,000 | 25 | 1.1 |
Sea nettle toxin (jellyfish) | 290,000 | 400 | 1.4 |
α-Toxin (Staphylococcus aureus) | 33,000 | 50 | 1.5 |
Parasitoxin (sea anemone) | 17,200 | 65 | 3.8 |
Rubescenslysin (mushroom) | 35,000 | 310 | 8.9 |
Hornetin (hornet venom) | 32,000 | 420 | 13 |
Cerebratulus III (marine worm) | 10,000 | 300 | 30 |
Epiactin B (sea anemone) | 19,500 | 750 | 38 |
Cardiotoxins (cobra venoms) | 7,000 | 1000 | 140 |
Pardaxin (fish) | 3,500 | 4600 | 1300 |
In this chapter, information about cytolytic toxins will be summarized. Particular attention will be paid to mechanisms of action, and to those toxins that have been obtained in a pure form. Other types of cytolytic toxins that act intracellularly to inhibit protein synthesis or to cause lipid peroxidation are not considered; more details of such toxins can be found in Chapter 3. Also, cytolytic toxins with phospholipase activity are mentioned here only briefly; information about phospholipases is contained in Chapter 2.
II. Biology of the Target Organ
All cells have plasma membranes that act as a barrier between the internal environment of the cell and the extracellular fluid. It follows, therefore, that all cells are potential targets for cytolytic toxins. Important differences do arise, however, because of differences in chemical composition of membranes in different cell types and because of different degrees of accessibility of some cells to toxins.
Plasma membranes are not just simple barriers that prevent loss of vital intracellular constituents; they are also specialised organelles that contribute to the characteristic properties of the cell. Membranes often maintain a significant electrochemical potential difference between the inside and outside of the cell; and membranes always participate in specialised biochemical reactions. The range of membrane properties is a consequence of the variations in the chemical constitution of the membrane. A brief summary of the composition of membranes will be given here. However, a full consideration of membrane biology is obviously outside the scope of this chapter. For an enjoyable introduction to this topic and for sources of more detailed information, see Robertson (1) and (2).
Biological membranes consist of a framework of lipids into which proteins are inserted. The lipid layer is only two molecules thick, hence the common term the lipid bilayer (FIG. 1). A bilayer is formed because of the tendency of the naturally occurring membrane lipids to aggregate in an organised manner. This is a consequence of the lipids having a hydrophobic tail formed by hydrocarbon chains and also hydrophilic head groups. The lipids of each half of the bilayer are arranged so that their hydrophobic tails meet in the center while their polar head groups are exposed to either the internal or external aqueous solutions. The lipid bilayer is a stable but flexible structure that is highly impermeable to ions and polar water-soluble molecules. Proteins can be loosely or tightly associated with the lipid bilayer (extrinsic proteins) on either the inside or the outside face, or they may span the lipid bilayer (intrinsic proteins). However, it must be remembered that there is still considerable mobility within membranes; the proteins are not fixed rigidly in a static bilayer. The membrane-bound proteins function as enzymes, ion channels, and receptors for hormones and neurotransmitters. The ratio of protein to lipid varies in different types of membrane. For example, in myelin there is about four times more lipid than protein, whereas in red blood cells there is slightly more protein than lipid.
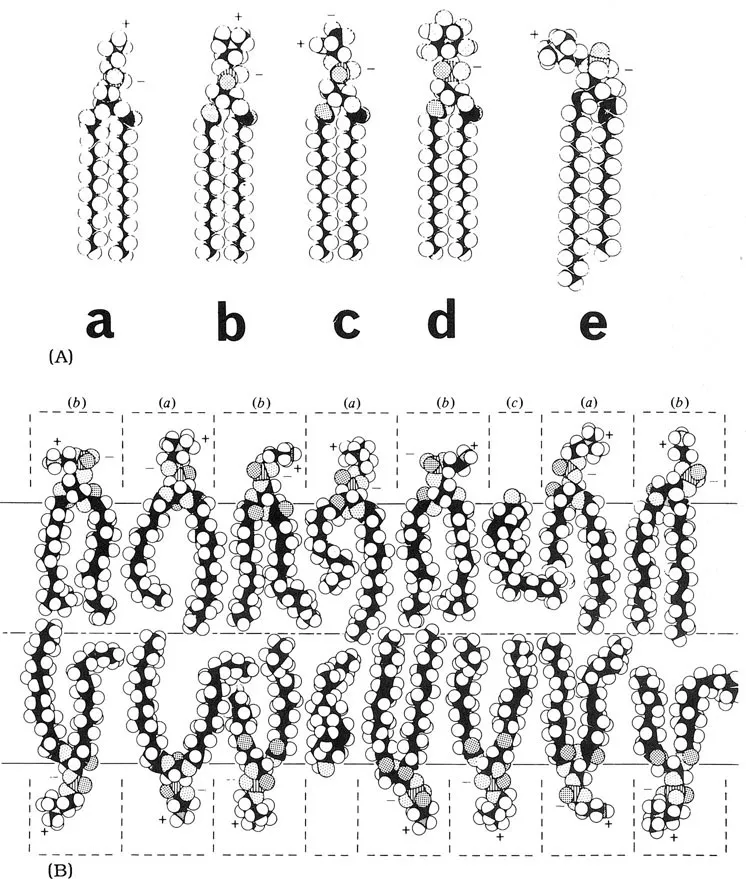
FIG. 1. Common membrane lipids. (A) Different phospholipids: a, phosphatidylethanolamine; b, phosphatidylcholine; c, phosphatidylserine; d, phosphatidylinositol and e, sphingomyelin. A molecule of water is shown to scale. (B) A bilayer of phosphatidylcholine, (a), phosphatidylethanolamine, (b), and cholesterol, (c). The molecules must be pictured as being in constant motion. The dotted lines draw attention to the effective spaces occupied by polar groups, kept apart by charge and bound water molecules. Space-filling atomic models are shown, with black carbons, white hydrogens, dotted oxygens (double dotted with a double bond), and hatched phosphorous atoms. Adapted from R.N. Robertson (1983) “The Lively Membranes” (Cambridge University Press), with permission.
Obviously, the protein content of cell membranes differs according to the cell type, but there is a surprising amount of variation in the composition of the lipids in different cell membranes. The major types of lipids are phospholipids, cholesterol, and glycolipids. The most common phospholipids are phosphatidylcholine, phosphatidylethanolamine, phosphatidyls...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Preface
- Table of Contents
- Contributor
- 1 Cytolytic Toxins
- 2 Phospholipases
- 3 Toxic Proteins Inhibiting Protein Synthesis
- 4 Enterotoxins
- 5 Toxins that Alter the Expression of Genetic Information: Genotoxins and Inhibitors of RNA or Protein Synthesis
- 6 Toxins Acting on the Cytoskeleton
- 7 Toxins Acting on Ion Channels and Synapses
- 8 Locally Acting Agents: Myotoxins, Hemorrhagic Toxins and Dermonecrotic Factors
- 9 Bacterial Endotoxins
- 10 Toxins Affecting Blood Coagulation and Fibrinolysis
- 11 Venom Components with Other Important Biological Activities
- Index