1 Methods in RNA interference
Martin Latterich and Dalia Halawani
Since the advent of DNA sequencing and polymerase chain reaction (PCR), there has been rarely one emerging technology that has received as much attention as the use of RNA interference (RNAi). The reason for this enthusiasm is that the phenomenon of RNAi firstly enabled a simple and inexpensive way to rapidly ablate specific messenger RNA (mRNA) species by inducing their degradation via a cellular protein machinery collectively named the RNA-induced silencing complex or RISC (Ketting et al., 2001). The phenomenon of RNAi was well known as a mechanism of inducing post-transcription gene silencing in plants and bacteria, where anti-sense RNA is being used to artificially silence the translation of proteins in select species. However, it is the discovery that small RNA polymers of 19–23 nucleotides can post-transcriptionally interfere with gene expression, either by inhibiting translation or inducing the degradation of complementary mRNA strands, that opened up applications in post-genomic research. For the first time it is now possible to synthesize small RNA species, as singlestranded, double-stranded or small hairpin structures, and introduce these molecules through common transfection methods into cells, where they serve to guide the RNA degradation machinery to the select target species. The RISC complex then systematically degrades the complementary mRNA, effectively resulting in the ablation of a specific mRNA species. Depending on the efficiency of ablation and the stability of the corresponding protein, the RNAi will ultimately result in the loss or reduction of the gene product. One of the major attractions of this technology is that it enables functional genetic analyses in eukaryotic systems that have been previously resilient to rapid genetic study, as well as gene ablation screens.
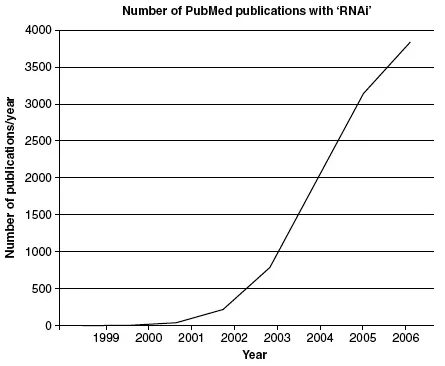
While the field of RNAi studies is relatively young, it has witnessed a rapidly expanding number of publications (Figure 1.1), with a remarkable 9000 publications in only 8 years. We predict that RNAi will not only remain an effective research tool to investigate gene function, but will soon be exploited in therapeutic applications to quench the expression of undesirable gene products. It is noteworthy that the recent Nobel Prize in Physiology or Medicine was awarded to Andrew Fire and Craig Mello for originally discovering and unraveling the molecular mechanism of double stranded RNAi (Fire et al., 1998), an outcome quite expected for a technique with such high impact.
The importance of RNAi and the wealth of published methods make it timely to compile a book with current techniques of use to the novice as well as expert user alike. We have decided to focus on some of the most common applications of RNAi, such as design, synthesis, and their introduction into different cell types and organisms, as well as some high throughput screening applications of emerging importance. While this book attempts to accurately convey current techniques, the methodologies are still evolving and innovation is much under way. We encourage the scientist user to handle the published methods as a basis for further experimentation and development. One key fact to remember is that while RNAi is a powerful tool, it has its limitations in terms of specificity and sensitivity. It is therefore necessary to validate the specificity of gene ablation using orthogonal approaches before reaching any conclusions.
Figure 1.1
Publication trend in research focusing on RNAi. A PubMed search, using ‘RNAi’ as keyword and restricted by year of publication was performed, and number of publications containing ‘RNAi’ were plotted against the year of their publication.
References
Fire, A., Xu, S., Montgomery, M.K., Kostas, S.A., Driver, S.E. and Mello, C.C. (1998) Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391: 806–811.
Ketting, R.F., Fischer, S.E., Bernstein, E., Sijen, T., Hannon, G.J. and Plasterk, R.H. (2001) Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Devel 15: 2654–2659.
2 RNAi reagent design
Bernd Jagla and Nathalie Aulner
2.1 Introduction
Over the last decade, RNAi has emerged as a key technology to study gene function and perform functional genomics studies. RNAi is a gene-specific knockdown technology in which the degradation of the target mRNA is guided by a homologous double-stranded RNA (dsRNA). Design considerations of RNAi reagents are the focus of this chapter.
RNAi is a well conserved process that is found throughout the eukaryotic kingdom. It is believed to be part of the cellular defense mechanism and, in the case of micro-RNAs (miRNAs), an integral part of post-transcriptional gene regulation (Ambros, 2001). Many studies have outlined the general mechanism: a long dsRNA is cut into small 21–23 base pair molecules termed small interfering RNAs (siRNA) by an intracellular endonuclease called Dicer (Ketting et al., 2001) before being loaded into RISC and targeting the specific degradation of an homologous mRNA (Martinez et al., 2002).
The discovery by Tuschl and colleagues that 21–23mer RNA duplexes can bypass mammalian cellular defense mechanisms against dsRNAs (Elbashir et al., 2001a) allowed the technology to quickly expand to mammalian cell systems. There are two major ways of introducing siRNAs into mammalian cells: (i) direct transfection of siRNA duplexes (chemically or enzymatically produced) and (ii) introduction of a vector driving the expression of short hairpin RNA (shRNA) that are further processed into siRNAs by Dicer. The double-stranded siRNA consists of a ‘guide strand’ or ‘guide’ and a ‘passenger strand’ or ‘passenger’. The guide strand binds to RISC forming activated RISC (RISC*) and the passenger strand is degraded.
To ensure the specificity and efficacy of the active siRNA molecules, it is pertinent to develop strategies for their rational design. Several sources of information are available that help finding rules and guidelines to successfully design siRNAs. Among these, studies of crystal structures of the RNAi machinery are one of the most revealing; they allow us to understand some of the properties that make a siRNA effective on a molecular level. Additionally, statistical analyses of active and non-active siRNA sequences are being used to infer siRNA design rules.
The risk of unspecific responses is an important consideration when designing RNAi reagents. Unspecific responses can be minimized by optimizing the sequence, experimental conditions, or chemically modifying the siRNA.
Algorithms based on considerations of RISC, sample sequences, and structural features of siRNA have been developed and some of them are available through web services and/or standalone programs. In this chapter, we describe what can be learned from recent structural studies of important players in the RNAi process, current design considerations, tools and databases available on the World Wide Web, and other related resources that help designing RNAi probes with a high probability of being active.
2.2 Lessons learned from X-ray structures/mechanism
The RISC is composed of Argonaute 2 and the single-strand guide. It is responsible for recognizing and processing dsRNAs into siRNAs, recognizing the target mRNA and processing it (Filipowicz, 2005).
Crystal structures of RNA silencing complexes provide insights into the recognition and cleavage machinery. From these structures we know that (i) the recognition of siRNAs requires a phosphorylated 5′-terminus of the guide strand; (ii) the 5′-terminal nucleotide is not bound to the complementary strand but rather interacts with RISC; (iii) the cleavage of the passenger and target RNA occurs at the position localized between nucleotides 10 and 11 on the guide strand; and (iv) the nucleotides of the 3′-end are more loosely connected to RISC and are most probably not important for target recognition (Ma et al., 2005; Parker et al., 2005).
As a practical consequence, it is desirable to have unique nucleotides between positions 2 and 11 of the guide, thus avoiding silencing unintended targets or introducing ‘off-target’ effects, and having an A, U (lower energy base pair) or unpaired nucleotide at the 5′-end of the guide strand.
Insights into RISC-mediated mRNA cleavage were obtained by analyzing the crystal structure of Archaeoglobus fulgidus Piwi protein (Af-Piwi) complexed with dsRNA (Ma et al., 2005; Parker et al., 2004) and Aquifex aeolicus Argonaute (Aa Ago) (Yuan et al., 2005). The Aa Ago protein is composed of four domains, an N-terminal domain, followed by a PAZ domain, a Mid domain, and a Piwi domain. The Piwi domain contains the mRNA cleavage site. The Mid domain contains the binding pocket for the 5′ phosphate of the guide strand, and the PAZ domain contains a 3′ overhang binding pocket. The process of guide strand-mediated mRNA binding, cleavage, and release within the context of the Ago scaffold in RISC comprises of a four-step catalytic cycle: nucleation, propagation, cleavage, and release (Yuan et al., 2005). The 5′-end of the guide strand is anchored within a highly conserved pocket formed by the Mid domain and the Piwi domain, making this nucleotide inaccessible for base pairing. This explains why nucleotides with low binding energies (A/U) are preferred at this position (Jagla et al., 2005). It also implies that this pocket is likely to be the site of 5′-end recognition of the guide strand and thus initiates nucleation. The Watson–Crick edges of nucleotides 2–8 (seed region) of the guide are directed outward into the solvent, providing access for target mRNA identification. Propagating the zippering up of the guide strand with target mRNA leads to the full-length duplex. Site-specific cleavage occurs between positions 10 and 11, as defined from the 5′-end of the guide strand, and uses a two-metal ion mechanism, like most type III ribonucleases (Parker et al., 2004). These structural data are highly correlated to experimental data; it has been proposed that base pairing between the 5′-end of the guide RNA and the mRNA is more critical than pairing at the 3′-end (Doench and Sharp, 2004). In addition, Haley and Zamore have shown that up to nine contiguous non-canonical pairs can be tolerated within the duplex segment towards the 3′-end of the guide strand (Haley and Zamore, 2004). This can be explained by crystal structure analysis showing that this region is not required for the formation of stable crystals (Yuan et al., 2005). Nonetheless, it appears that proper base pairing contributes to the catalytic rate (Haley and Zamore, 2004). Release of the cleaved product closes the cycle and frees RISC up to silence the next mRNA.
2.3 Current design considerations
Apart from the considerations that can be derived from the crystal structures, statistical analysis of experimentally verified functional and nonfunctional siRNAs can provide insights into the design of effective siRNAs. Important considerations for designing RNAi reagents are: asymmetry, positional preferences of certain nucleotides within the sequence, nonsequence position-based considerations, practical considerations relating to synthesis or experimental setup, and specificity. In the following section, we describe these design considerations in more detail. How to improve siRNAs by chemical modification is described elsewhere in this book (Chapter 3).
2.3.1 Asymmetry
A dsRNA is asymmetric if one side of the molecule has a higher binding energy than the other, that is, contains more G/C. When designing RNAi reagents, the 5′-end of the guide strand should have a lower binding energy than the 3′-end (Schwarz et al., 2003). More specifically, the nucleotide at the 5′-end of the guide strand should be A, U or even could be unpaired with the target or the passenger strand. This will favor one strand to assemble into RISC and trigger the interference. It was shown that by changing the symmetry of an inactive siRNA, efficacy was increased (Hutvagner, 2005; Khvorova et al., 2003; Schwarz et al., 2003). Moreover, statistical analysis has shown that functional siRNA duplexes have a lower internal stability at the 5′-end of the guide than that of non-functional duplexes (Khvorova et al., 2003; Reynolds et al., 2004). Another way to ensure this asymmetry is to apply a combination of rules, based on statistical analysis. For example, work from our lab and those from Ui-Tei and colleagues have shown that at least five A or U residues in the 5′-terminal third of the guide strand are required (Jagla et al., 2005; Ui-Tei et al., 2004). In conjunction with a medium-high G/C content, this means that the 5′-end of the guide strand needs to have a higher A/C content than the 3′-end. Other flavors are described by Santoyo et al. (2005), w...