A1 Acanthamoeba spp.
Naveed Ahmed Khan
1 Introduction
Acanthamoeba is an opportunistic protozoan pathogen, a member of free-living amoebae that are widely distributed in the environment. During the last few decades, Acanthamoeba has become an increasingly important microbe. It is now well-recognized as a human pathogen causing serious as well as life-threatening infections, has a potential role in ecosystems, and acts as a carrier and a reservoir for prokaryotes and viruses.
2 Discovery of pathogenic free-living amoebae
The term ‘amoebae’ encompasses the largest diverse group of protist organisms that have been studied since the discovery of the early microscope, the largest being Amoeba proteus (Figures 1 and 2). Although these organisms have a common amoeboid motion, that is a crawling-like movement, they have been classified into several different groups. These include potent parasitic organisms such as Entamoeba spp. that were discovered in 1873 from a patient suffering from bloody dysentery and named E. histolytica in 1903. Among free-living amoebae, Naegleria was first discovered by Schardinger in 1899, who named it Amoeba gruberi. In 1912, Alexeieff suggested its genus name Naegleria, and much later, in 1970, Carter identified Naegleria fowleri as the causative agent of fatal human infections (reviewed in De Jonckheere, 2002). In 1913, Puschkarew isolated an amoeba from dust, and later in 1931, the genus Acanthamoeba was created (Castellani, 1930; Douglas, 1930; Volkonsky, 1931). Balamuthia mandrillaris has only been discovered relatively recently in 1986 from the brain of a baboon that died of meningoencephalitis and was described as a new genus, Balamuthia (Visvesvara et al., 1990, 1993). Over the years, these free-living amoebae have gained increasing attention from the scientific community due to their diverse roles, in particular, in causing serious and sometimes fatal human infections (Figure 3).
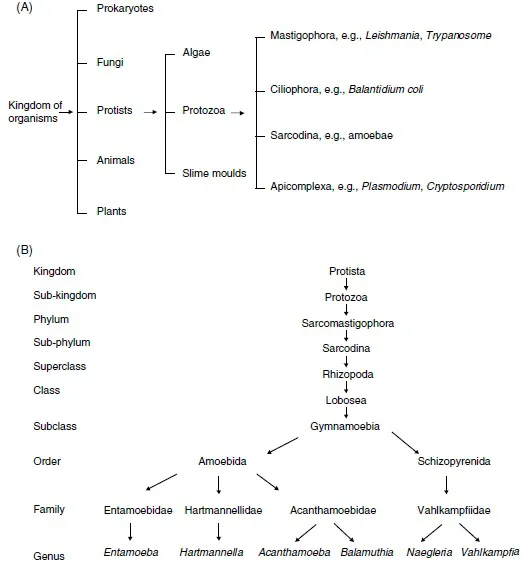
Figure 1 The traditional classification scheme of (A) protozoan pathogens, and (B) free-living amoebae, based largely on morphological characteristics.
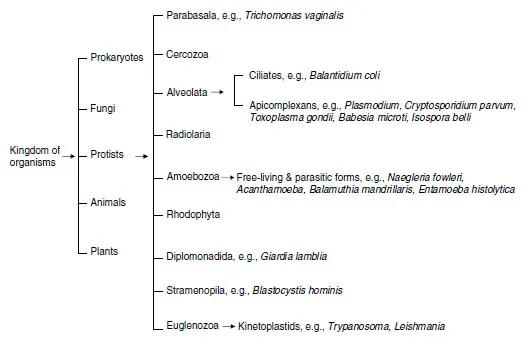
Figure 2 The present classification scheme of protozoan pathogens, based largely on their genetic relatedness.
3 Acanthamoeba spp.
In 1913, Puschkarew isolated an amoeba from dust and named it Amoeba polyphagus. Later in 1930, Castellani isolated an amoeba that occurred as a contaminant in a culture of the fungus Cryptococcus pararoseus (Castellani, 1930). These amoebae were round or oval in shape with a diameter of 13.5–22.5 μm and exhibited the presence of pseudopodia (now known as acanthopodia). In addition, the encysted form of these amoebae exhibited double walls with an average diameter of between 9 and 12 μm. This amoeba was placed in the genus Hartmannella, and named Hartmannella castellanii. A year later, Volkonsky subdivided the Hartmannella genus into three genera (Volkonsky, 1931), based on the following characteristics:
- Hartmannella: amoebae characterized by round, smooth-walled cysts were placed in this genus.
- Glaeseria: this genus included amoebae characterized by nuclear division in the cysts.
- Acanthamoeba: amoebae characterized by the appearance of pointed spindles at mitosis, and double-walled cysts and an irregular outer layer were placed in this genus.
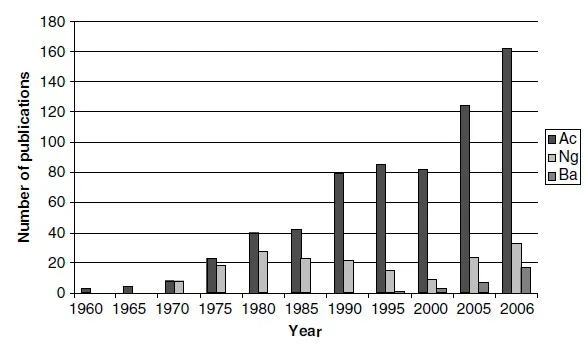
Figure 3 The number of published articles on free-living amoebae. Data for Acanthamoeba (Ac), Naegleria (Ng) and Balamuthia (Ba) were collected from PubMed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi).
Singh (1950) and Singh and Das (1970) argued that the classification of amoebae by morphology, locomotion and appearance of cysts was of limited phylogenetic value and that these characteristics were not diagnostic. They concluded that the shape of the mitotic spindle was inadequate as a generic character and discarded the genus Acanthamoeba. In 1966, Pussard agreed with Singh (1950) that the spindle shape was an unsatisfactory feature for species differentiation but considered the distinctive morphology of the cyst to be a decisive character at the generic level and recognized the genus Acanthamoeba. After studying several strains of Hartmannella and Acanthamoeba, Page (1967a, 1967b) also concluded that the shape of the spindle was a doubtful criterion for species differentiation. He considered the presence of acanthopodia and the structure of the cyst to be sufficiently distinctive to justify the generic designations of Hartmannella and Acanthamoeba. He also stated that the genus Hartmannella had nothing in common with Acanthamoeba except for a general mitotic pattern, which is a property shared with many other amoebae.
In 1975, Sawyer and Griffin created the family Acanthamoebidae and Page (1988) placed Hartmannella in the family Hartmannellidae. The current position of Acanthamoeba in relation to Hartmannella, Naegleria and other free-living amoebae is shown in Figures 1 and 2.
The prefix ‘acanth’ (Greek meaning ‘spikes’) was added to the term amoeba to indicate the presence of spine-like structures (now known as acanthopodia) on the surface of this organism. After the initial discovery, this organism was largely ignored for several decades. However, in the late 1950s, it was discovered as a tissue culture contaminant (Culbertson et al. , 1958; Jahnes et al., 1957). Later, Culbertson and colleagues (Culbertson et al., 1958, 1959), for the first time, demonstrated its pathogenic potential by exhibiting its ability to produce cytopathic effects on monkey kidney cells in vitro, and to kill laboratory animals in vivo. The first clearly identified Acanthamoeba granulomatous encephalitis (AGE) in humans was observed in 1972 (Jager and Stamm, 1972). Acanthamoeba keratitis cases were reported for the first time in the early 1970s (Jones et al., 1975; Nagington et al., 1974). Acanthamoeba was first shown to be infected with bacteria in 1954 (Drozanski, 1956), demonstrated to harbour bacteria as endosymbionts in 1975 (Proca-Ciobanu et al., 1975) and later identified as a reservoir for pathogenic facultative mycobacteria (Krishna-Prasad and Gupta, 1978). Acanthamoeba was first linked with Legionnaires’ disease by Rowbotham (1980). Since then the worldwide research interest in the field of Acanthamoeba has increased dramatically and continues to do so (Figure 3).
3.1 Ecological distribution
Acanthamoeba has the ability to survive in diverse environments and has been isolated from public water supplies, swimming pools, bottled water, sea water, pond water, stagnant water, fresh-water lakes, salt-water lakes, river water, distilled water bottles, ventilation ducts, the water-air interface, air-conditioning units, sewage, compost, sediments, soil, beaches, vegetables, surgical instruments, contact lenses and their cases, and from the atmosphere with the recent demonstration of Acanthamoeba isolation even by air sampling, indicating the ubiquitous nature of this organism. In addition, Acanthamoeba has been recovered from hospitals, dialysis units, eye-wash stations, human nasal cavities, pharyngeal swabs, lungs tissues, skin lesions, from bone graft of the mandible suffering from osteomyelitis, corneal biopsies, cerebrospinal fluids (CSF) and brain necropsies (reviewed in Khan, 2003; Marciano-Cabral and Cabral, 2003; Schuster and Visvesvara, 2004; Visvesvara et al., 2007). It is not surprising that the majority of healthy individuals have been shown to possess anti- Acanthamoeba antibodies indicating our common exposure to this organism (Cursons et al., 1980).
3.2 Biology
The vegetative state of Acanthamoeba is known as trophozoites. Trophozoites are normally in the range of 12–35 μm in diameter; however the size varies significantly between isolates belonging to different species/genotypes. In general, Acanthamoeba does not differ greatly at the ultrastructural level from a mammalian cell, and thus presents an excellent model for cell biology studies. The trophozoites exhibit spine-like structures on their surface known as acanthopodia. The acanthopodia are most likely important in adhesion to surfaces (biological or inert), cellular movements or capturing prey. The acanthopodia are composed of hyaline (transparent) cytoplasm, which excludes various vacuoles and particles that are normally present on the interior of the cell (Bowers and Korn, 1968). The trophozoites normally possess a single nucleus that is approximately one-sixth the size of the trophozoite. The nucleus possesses a prominent nucleolus (approximately 2.4 μm in diameter, occupying oneeighth of the nuclear volume, while two nucleoli per nucleus are not uncommon), but multinucleate amoebae have been observed. The membranes of the nucleus are separated by a distance of about 350 Å (Bowers and Korn, 1968). There are numerous nuclear pores that are about 1040 Å in diameter. RNA synthesis in Acanthamoeba is a nuclear function. As for other eukaryotic cells, Acanthamoeba possess an extensive network of both smooth and rough endoplasmic reticulum (ER), with ribosomes stubbed on the cytoplasmic surface of the rough ER (Bowers and Korn, 1968). These ribosomes translate messenger RNA and actively synthesize proteins. However, not all proteins are synthesized on the ER-bound ribosomes. Protein synthesis also occurs on the unbound ribosomes that are free in the cytosol. Generally speaking, secretory proteins and membrane proteins are made by ER-bound ribosomes, while proteins intended for use within the cytosol are made on free ribosomes. The pattern of ribosomal proteins analyzed by 2-dimensional gel electrophoresis revealed that the small subunit of cytoplasmic ribosome contains 25 proteins, while the large subunit contains 40 proteins. Furthermore, exponentially growing Acanthamoeba contains a 45-kDa phosphorylated small subunit ribosomal protein that is absent in the cyst ribosomes. In contrast, smooth ER does not possess ribosomes and thus has no protein synthesis. Smooth ER is involved in the synthesis of lipids and may play a role in the inactivation or detoxification of drugs that might otherwise be toxic or harmful to the cell. Proteins from the ER-bound ribosomes bud off the ER in vesicles and arrive at the Golgi complex (named after its Italian discoverer, Camillo Golgi), which plays an important role in the synthesis of complex polysaccharides. Here, proteins (and other substances) undergo posttranslational modifications (most notably glycosylation). Carbohydrates are shown to be either linked to the nitrogen atom of an amino group (N-linked glycosylation, that is shown to be limited to the asparagine amino acid) or to the oxygen atom of a hydroxyl group (O-linked glycosylation, i.e., limited to serine or threonine amino acids but may also be attached to hydroxylysine or hydroxyproline, which are derivatives of the amino acids lysine and proline, respectively). The modified contents are then passed on to other compartments of the cell by means of vesicles that arise by budding off the Golgi complex. The process of glycosylation initiates within the lumen of the ER and is completed within the Golgi complex and its products are finally destined for the cell membrane or for export.
Under the microscope, an actively feeding trophozoite exhibits one or more prominent contractile vacuoles (contractility meaning shortening), whose function is to expel water (i.e., water expulsion vacuoles), and which are involved in osmotic regulation. Usually, these do not contain any precipitates but exhibit alkaline phosphatase activity in the membrane; this activity may also be present in other vesicles (Bowers and Korn, 1974). During discharge, a contractile vacuole becomes associated with the plasmalemma and may account for the alkaline phosphatase activity in the plasma membrane. In addition, it is shown that alkaline phosphatase activity in the plasma membra...