
eBook - ePub
Human Embryonic Stem Cells
- 391 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Human Embryonic Stem Cells
About this book
Since the first successful isolation and cultivation of human embryonic stem cells at the University of Wisconsin, Madison in 1998, there has been high levels of both interest and controversy in this area of research.This book provides a concise overview of an exciting field, covering the characteristics of both human embryonic stem cells and pluripotent stem cells from other human cell lineages. The following chapters describe state-of-the-art differentiation and characterization of specific ectoderm, mesoderm and endoderm-derived lineages from human embryonic stem cells, emphasizing how these can be used to study human developmental mechanisms. A further chapter discusses genetic manipulation of human ES cells. The concluding section covers therapeutic applications of human ES cells, as well as addressing the ethical and legal issues that this research have raised.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
Ciencias biológicasSubtopic
Bioquímica1. | Biology of embryonic stem cells |
1.1 Introduction
The first reports of cultured pluripotent mouse embryonic stem (ES) cell lines appeared more than 20 years ago (Evans and Kaufman, 1981; Martin, 1981). Early optimism that equivalent cell lines could be established from other mammalian species was not realized and until recently the mouse remained the only mammalian species from which stable pluripotent cell lines could be isolated. Accordingly, mouse ES cells have become the paradigm for the study and exploitation of mammalian stem cells. The greatest impact of ES cells over the last 20 years has been the development of gene targeting technology, in which ES cells provide a vector for the creation and analysis of precise alterations to the mouse genome. Experimental analysis and manipulation of ES cells has also increased our knowledge of the biochemical basis of pluripotence and extended our understanding of embryogenesis, principally in the processes of differentiation and cell fate determination. The lessons learned from mouse ES cells will inform the characterization of the recently established human ES cell lines (Thomson et al., 1998; Reubinoff et al., 2000), and the application of these cells to the study of human development and treatment of human disease.
1.2 Derivation and definition of mouse ES cells
The inner cell mass of the mouse blastocyst at about 4 days post-coitum is normally fated to form all cells and tissues of the embryo and extraembryonic yolk sac. Mouse ES cells were originally derived from this population of 20–40 pluripotent cells by culturing whole blastocysts or the surgically removed inner cell mass on a feeder layer of mitotically inactivated mouse embryonic fibroblasts (Brook and Gardner, 1997; Evans and Kaufman, 1981; Hogan et al., 1994; Martin, 1981). More recently, successful culture has been performed in medium supplemented with one of a number of cytokines from the interleukin 6 (IL-6) family (Nichols et al., 1990, 1994; Pease et al., 1990). After several days, proliferating cells are disaggregated and replated. Colonies with the undifferentiated morphology characteristic of pluripotent ES cells (Figure 1.1) can be selected and propagated clonally by disaggregating to a single-cell suspension and reseeding. Successful derivation of ES cells has been achieved using blastocysts obtained from particular inbred strains of mice, principally 129 and C57BL/6 (Brook and Gardner, 1997; Evans and Kaufman, 1981; Hogan et al., 1994; Kaufman et al., 1983; Martin, 1981), but derivation from other strains has proven problematic.
1.2.1 Pluripotency of ES cells
A number of different approaches show that ES cells are pluripotent and can differentiate to form cell populations derived from all three primary germ layers, endoderm, ectoderm, and mesoderm. These include (1) directed and random differentiation of ES cells in culture (Bain et al., 1995; Doetschman et al., 1985; Guan et al., 1999; Lake et al., 2000; Nakano et al., 1994; Wobus et al., 1984), (2) implantation under the kidney capsule of adult mice resulting in the formation of teratomas (Damjanov et al., 1987; Kaufman et al., 1983), and (3) introduction into the mouse morula or blastocyst resulting in chimeras in which the ES cells contribute to all fetal and adult tissues including germ-line cells (Beddington and Robertson, 1989; Bradley et al., 1984; Lallemand and Brulet, 1990; Smith, 1992; Wood et al., 1993).
Although usually considered pluripotent, ES cells may be totipotent since in chimeras they sometimes contribute to extraembryonic visceral endoderm, the parietal endoderm of the yolk sac and also, albeit rarely, to the trophoblast-derived placenta (Beddington and Robertson, 1989). Consistent with this, ES cells in culture readily form parietal and visceral endoderm (Lake et al., 2000), and a 50% reduction in the expression of the POU transcription factor Oct4 is sufficient to convert ES cells to trophoblast cells (Niwa et al., 2000). These data suggest that in the appropriate environment ES cells are capable of forming all tissues of the embryo and adult, including all extraembryonic tissue.
1.2.2 Self-renewal of ES cells
In vitro, ES cells grow as domed colonies (Figure 1.1) which can proliferate without differentiation in medium supplemented with either leukemia inhibitory factor (LIF) (Pease and Williams, 1990; Williams et al., 1988) or one of a number of cytokines from the IL-6 family (Conover et al., 1993; Nichols et al., 1994; Pennica et al., 1995; Rose et al., 1994; Yoshida et al., 1994). These cytokines signal through the gp130 receptor subunit (Chow et al., 2002) and are both necessary and sufficient for the isolation and maintenance of ES cells (Nichols et al., 1990, 1994; Pease et al., 1990). Support of ES cells by more complex culture environments, such as medium supplemented with Buffalo Rat Liver conditioned medium or by co-culture with embryonic fibroblasts, has been shown to be dependent on the paracrine supply of LIF (Rathjen et al., 1990a,b; Smith et al., 1988).
In the presence of LIF, ES cells retain an indefinite capacity for self-renewal without transformation, and their growth is not restricted by contact inhibition or proliferative senescence. Compared with cancer cells, ES cells remain substantially normal karyotypically over extended culture. However, many ES cell lines contain subpopulations of cells with chromosomal abnormalities. Trisomy-8 ES cells have a selective growth advantage over euploid cells and rarely contribute to germ-line transmission in chimeras (Liu et al., 1997b). Increasing passage number can result in aneuploidy, which correlates with reduced efficiency of both chimera formation and germ-line transmission (Longo et al., 1997).
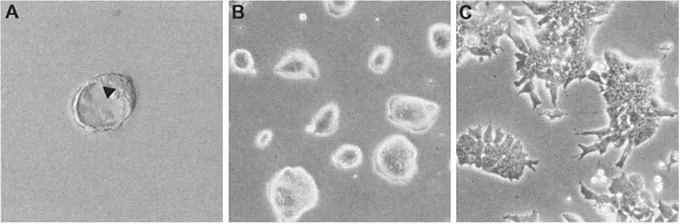
Figure 1.1: (A) 3.5-day post-coitum mouse blastocyst. The arrow indicates the inner cell mass (ICM), a population of pluripotent cells localized at one pole of the blastocelic cavity. The image was taken using Hoffman interference contrast and a 20× objective. (B) Mouse ES cells in culture, growing in characteristic domed, three-dimensional colonies in which individual cells cannot be discerned. (C) ES cells cultured in HepG2 conditioned medium differentiate to form a homogeneous population of EPL cells. Differentiation is accompanied by alterations in colony morphology, with cells growing in two-dimensional sheets in which individual cells can be easily observed. (B) and (C) were photographed using phase contrast and a 20× objective.
1.2.3 Markers of ES cells
Aside from their pluripotency and self-renewing properties, undifferentiated mouse ES cells can be characterized by a number of markers (Table 1.1), some of which are known to be essential for ES cell survival and self-renewal (see below) and almost all of which are known to be expressed in inner cell mass cells of the embryo. Within ES and inner cell mass cells, alkaline phosphatase activity is high (Johnson et al., 1977; Matsui et al., 1992; Mulnard and Huygens, 1978; Wobus et al., 1984) as is telomerase activity (Armstrong et al., 2000; Liu et al., 2000b), the latter being consistent with the self-renewal properties and genomic stability of pluripotent cells. The expression of most of these markers and activities is not exclusive to pluripotent cells but in combination their expression appears to define mouse ES cells uniquely.
1.3 The molecular basis of ES cell pluripotency
Several genes and signaling pathways have been identified as important for survival and maintenance of pluripotence of ES cells in culture and inner cell mass cells in the embryo.
1.3.1 Transcriptional regulators
A key regulator of pluripotence is the POU transcription factor Oct4, also referred to as POU5f1, Oct3 or Oct3/4 (Niwa et al., 2000, 2002). Oct4 is expressed in ES cells (Nichols et al., 1998) and continues to be expressed as these cells differentiate to early primitive ectoderm-like (EPL) cells, an in vitro equivalent of primitive ectoderm in the embryo at ~5.5 days post-coitum (Figure 1.1; Pelton et al., 2002). Oct4 expression is downregulated as ES cells differentiate further to cells representative of the three germ layers (Palmieri et al., 1994; Rathjen et al., 1999).
Table 1.1: Markers of ES cells.
Marker | Type of protein | Expressed in ICM? | References |
SSEA-1a | Cell-surface antigen | Yes | (Matsui et al., 1992; Solter and Knowles, 1978) |
Fgf4 | Growth factor | Yes | (Niswander and Martin, 1992; Rappolee et al., 1994; Yuan et al., 1995) |
Tpoa | Growth factor | Not known | (Xie et al., 2002) |
Esg-1a | RNA binding? | Yes | (Bierbaum et al., 1994; Tanaka et al., 2002) |
Psc1 | mRNA regulation? | Yes | (Pelton et al., 2002) |
CRTR-1 | CP2 TFa | Yes | (Pelton et al., 2002) |
Foxd3b | Forkhead TF | Yesd | (Hanna et al., 2002) |
Gbx2 | Homeobox TF | Yes | (Chapman et al., 1997) |
Oct4c | POU TF | Yes | (Nichols et al., 2001; Niwa et al., 2000; Pelton et al., 2002) |
Rex1 | Zinc-finger TF | Yes | (Pelton et al., 2002; Rogers et al., 1991) |
Sox2 | SRY-related HMG Box TF | Yes | (Avilion et al., 2003; Tomioka et al., 2002) |
Taube nuss | Transcription coactivator? | Yes | (Voss et al., 2000) |
a Abbreviations: SSEA-1, stage-specific embryonic antigen-1; Tpo, thrombopoietin; TF, transcription factor; Esg-1, embryonal stem cell-specific gene-1.
b Hfh2 or genesis.
c POU5f1, Oct3 or Oct3/4.
d Detected in whole blastocysts and 6.5 days post-coitum epiblast.
The pluripotency of mouse ES cells appears to depend on tightly regulated Oct4 expression. Using a tetracycline-regulated Oct4 transgene, Niwa et al. (2000) showed that a reduction of 50% or more in expression induced differentiation of ES cells to extraembryonic trophoblast cells, whilst a 50% increase in Oct4 expression triggered differentiation to endoderm and mesoderm. Production of primitive endoderm in F9 embryonal carcinoma cells stimulated by retinoic acid treatment is also accompanied by a transient increase in Oct4 expression (Botquin et al., 1998).
The expression and function of Oct4 in the early embryo is also consistent with a requirement...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Contributors
- Abbreviations
- Foreword
- Preface
- 1. Biology of embryonic stem cells
- 2. Characteristics of human embryonic stem cells, embryonal carcinoma cells and embryonic germ cells
- 3. Adult stem cell plasticity
- 4. Human and murine embryonic stem cell lines: windows to early mammalian development
- 5. Human mesenchymal stem cells and multilineage differentiation to mesoderm lineages
- 6. Trophoblast differentiation from embryonic stem cells
- 7. Current and future prospects for hematopoiesis studies using human embryonic stem cells
- 8. Derivation of endothelial cells from human embryonic stem cells
- 9. Neural specification from human embryonic stem cells
- 10. Modeling islet development through embryonic stem cell differentiation
- 11. Cardiomyocyte differentiation in human embryonic stem cell progeny
- 12. Genetic engineering of human embryonic stem cells
- 13. ES cells for transplantation: coping with immunity
- 14. Clinical applications for human ES cells
- 15. Production of human embryonic stem cell-derived cellular product for therapeutic use
- 16. Ethical and policy considerations in embryonic stem cell research
- 17. Legal framework pertaining to research creating or using human embryonic stem cells
- 18. Genomic approaches to stem cell biology
- 19. Proteomics and embryonic stem cells
- Appendix: Human embryonic stem cell resources
- Index
- Color plates can be found between p. 136 and p. 137.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Human Embryonic Stem Cells by Jon Odorico, Roger Pedersen, Su-Chun Zhang, Jon Odorico,Roger Pedersen,Su-Chun Zhang in PDF and/or ePUB format, as well as other popular books in Ciencias biológicas & Bioquímica. We have over one million books available in our catalogue for you to explore.