
- 352 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Bone Tissue Engineering
About this book
Focusing on bone biology, Bone Tissue Engineering integrates basic sciences with tissue engineering. It includes contributions from world-renowned researchers and clinicians who discuss key topics such as different models and approaches to bone tissue engineering, as well as exciting clinical applications for patients. Divided into four sections, t
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
SECTION II
Basic Bone Biology and Scaffold Designs for Tissue
4 The Organic and Inorganic Matrices
Adele L Boskey
0-8493-1621 -9/05/$0.00+$ 1.50
© 2005 by CRC Press LLC
© 2005 by CRC Press LLC
INTRODUCTION
The structure and composition of bone varies with the tissue site and its origin (e.g., lamellar versus cortical, intramembranous versus endochondral) as well as with age, diet, and health status. Engineering bone requires mimicking of key aspects of bone structure and composition to achieve the optimal functional tissue. To appreciate these characteristics, this chapter will review current knowledge of the functions of the mineral and matrix constituents, and how these functions have been assessed by studies of age and disease variations in tissue composition. Some comment will also be made on the current state of bone tissue engineering from the mineral and matrix points of view. The functions of the bone cells and their importance in bone tissue engineering were discussed in chapter ([0-9]+).
Tissue-engineered bone and other mineralized tissues will be important for the repair of lesions caused by cancer surgery, birth defects, and trauma. Since bone is unique in terms of being able to repair itself1 (e.g., fracture healing in humans and animals, limb regeneration in lower species), lessons can not only be learned by examination of these processes, but there may be instances when tissueengineered products may only be used to enhance the natural repair process.
BONE AS A COMPOSITE
Bone is a composite material consisting of mineral, matrix, cells, and water. Developmentally as the cartilaginous anlage of the bone shaft is replaced by a boney matrix, that matrix is predominantly matrix (osteoid) and calcified cartilage.2 With age the mineral content increases, reaching a maximum value, approximated by “peak bone density,” in male and female humans at different ages.3
In general, as the animal matures even further the total bone mineral content decreases.4 Diseases such as osteoporosis are associated with a decrease in total bone density, but not necessarily with decreases in the proportion of mineral in any bone, while osteomalacia is defined as a loss of bone mineral and an increase in osteoid.5 Conversely, osteopetrosis is an increase in both bone mineral and bone matrix, beyond that necessary for normal function.6 Like many other composite materials, the integration of minerals within the organic matrix enables bone to have mechanical properties that are enhanced relative to the properties of the mineral (a brittle material) or the matrix (an elastic material) alone.
Bone has both mechanical and homeostatic functions, providing protection for the internal organs of the body and serving as a storage site and source of mineral ions.7 In terms of evolution, the vertebrates developed calcium phosphate skeletons, but there are lower species with other mineralized exo-and endo-skeletons that contain calcium carbonates and other phases. The composition of those species’ skeletons are reviewed elsewhere, along with discussions of how the study of these species can contribute to the development of tissue-engineered products.8–12 Bone strength is determined by the shape of the bone, the structure (arrangement of its components), that is, its geometry, and by its so-called “material” properties.7,13 The stress-strain curve of the bone (Figure 4.1) has areas that have been shown to be determined predominantly by the mineral phase (elastic region) and principally by the organic phase (plastic region).14 The slope of the stress-strain curve is the elastic (Young’s) modulus, which describes the intrinsic stiffness of the bone. Hence, all other components being equal, a more highly mineralized material will have a greater elastic modulus. The area under the stress-strain curve is the amount of energy needed to cause a fracture; thus, bones with comparable Young’s moduli that have stress-strain curves extended into the plastic region will be more resistant to load than those that do not.
There is a well-established correlation between bone mineral density (BMD) measured radiographically and bone strength, but BMD does not completely account for bone strength.15 Rather, it is other properties of the composite tissue that are believed to explain this variation.
THE ORGANIC MATRIX
COLLAGEN
Type I collagen is the principal component of the organic matrix of bone, accounting for approximately 30% of the dry nondemineralized matrix. Type I collagen is a heteropolymer of two identical and one distinct chain, each having the primary structure (Gly–X–Y) n, where X and Y are frequently proline or hydroxyproline. The collagen is post-translationally modified to contain hydroxylysine, hydroxyproline, and glycoslyated hydroxylysine.16 In the extracellular matrix, the hydroxylysine residues are involved in the formation of stable collagen cross-links17 (Figure 4.2).
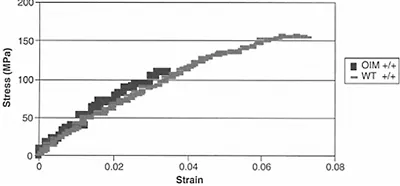
Figure 4.1 Theoretical stress-strain curve showing the elastic and plastic regions defined by the yield point. The dotted line represents typical data, and the straight lines represent the slopes used to calculate various parameters.
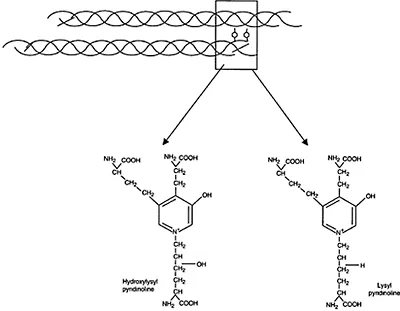
Figure 4.2 Illustration of trivalent collagen cross-links formed between collagen triple helical molecules (box). The structures of the two major stable cross-links, hydroxylysyl pyridinoline and lysyl pyridinoline, are illustrated.
In addition, bone contains small amounts of type III, type V, and type XII collagen. These collagens trim the type I fibrils, and may affect the properties of the tissue. Thus, in the fro/fro mouse,18 a naturally occurring mutant with decreased type III collagen, the bones are deformed, undermineralized, and fragile, and the proportion of collagen fibrils with a large diameter is decreased (in the tendon). This mutant also has decreased levels of the noncollagenous protein, osteonectin (see below). Mice engineered with a type V collagen mutation have thinner fibrils, skeletal deformities, and abnormalities in all the type I-containing tissues.19
Type I collagen provides a backbone for the deposition of bone mineral. The bone mineral crystals are aligned with their long axis parallel to the collagen axis. Where the collagen molecule is altered, as in the case of the genetic abnormalities in osteogenesis imperfecta, otherwise known as brittle bone disease, mineral crystals are generally smaller in size than those in age-matched healthy bone20 and the mineral may be found outside the collagen fibrils.21 The altered bone and mineral properties in this collagen-based disease are the best evidence for proving the importance of the collagen for proper bone mineralization. Figure 4.3 shows the stress-strain curve for bones from oim/oim (osteogenesis imperfecta mouse) bones and those of age-matched wild-type animals, showing the increased brittle character of the animals, which have a type I-homotrimer collagen as opposed to the heteropolymer in the wild-type animals. The collagen gives bone its elastic properties, and there have been several studies demonstrating that the connective tissues, as well as the bone, in animal models of osteogenesis imperfecta are brittle (i.e., absorbs little energy before failure), and fails more easily when load is applied.22,23
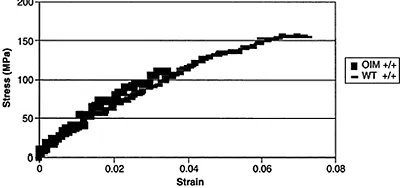
Figure 4.3 Measured stress-strain curves from 4-month-old osteogenesis imperfecta (oim/oim) mice and the age-matched controls. Note that the oim/oim mice (darker symbols) have no plastic region, and a yield point lower than the wildtype (lighter symbols), and thus have a decreased ability to bear load, and are thus more brittle. Courtesy of Dr. Nancy Camacho.
But collagen itself is not the bone matrix component that facilitates mineral formation (the process of nucleation by which the initial mineral forms will be discussed in section IV of this chapter). This was demonstrated many years ago when it was noted that when decalcified bone collagen was implanted in situ, it took many months for mineral deposition to commence.24 Termine et al. later demonstrated that it was the other proteins associated with the bone collagen matrix that were facilitating bone mineralization.25 Similarly, Glimcher and Endo showed that the ability to mineralize collagen in vitro was directly related to the extent to which its associated proteins were phosphorylated.26 Thus, it is apparent that bone collagen re-mineralization requires, at a minimum, phosphorylated noncollagenous proteins.
BONE MATRIX PROTEINS
The noncollagenous proteins account for approximately 5% of the dry bone matrix; yet, it is these proteins that appear to regulate the organization, turnover, and mineralization of the bone matrix. Most of these proteins appear to have more than one function. However, because their functions in humans have been obtained from investigations in vitro, or in transgenic animals, the only proof of function comes from the limited patient data available. These proteins fall into a series of families, and their functions appear to be redundant. This redundancy is crucial, as the presence of a mineralized skeleton is a key feature of a viable vertebrate, and were the functional proteins not redundant in activity, it would be impossible to maintain a functional tissue.
SIBLINGs
The SIBLING proteins (Small Integrin Binding LIgand with N-Glycosylation) are all located on human chromosome 4, all have one or more arginine-glycine-aspartate (RGD) sequences, and all have som...
Table of contents
- COVER PAGE
- TITLE PAGE
- COPYRIGHT PAGE
- PREFACE
- EDITORS
- CONTRIBUTORS
- SECTION I: BASIC BONE BIOLOGY AND TISSUE ENGINEERING
- SECTION II: BASIC BONE BIOLOGY AND SCAFFOLD DESIGNS FOR TISSUE
- SECTION III: APPLIED PRINCIPLES OF BONE TISSUE ENGINEERING
- SECTION IV: CLINCAL OPPORTUNITIES
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Bone Tissue Engineering by Jeffrey O. Hollinger,Thomas A. Einhorn,Bruce Doll,Charles Sfeir in PDF and/or ePUB format, as well as other popular books in Medicina & Biotecnología en medicina. We have over one million books available in our catalogue for you to explore.