
- 552 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Electrogenerated Chemiluminescence
About this book
The first source on this expanding analytical science, this reference explores advances in the instrumentation, design, and application of techniques with electrogenerated chemiluminescence (ECL), examining the use and impact of ECL-based assays in clinical diagnostics, life science research, environmental testing, food and water evaluation, and th
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction
Allen J. Bard
The University of Texas at Austin, Austin, Texas, U.S.A.
The University of Texas at Austin, Austin, Texas, U.S.A.
I. BACKGROUND AND SCOPE
This monograph deals with light emission at electrodes in electrochemical cells caused by energetic electron transfer (redox) reactions of electrogenerated species in solution. A typical system would involve a solution containing reactants A and D in a solution with supporting electrolyte, for example, MeCN with tetra-n-butylammonium perchlorate (TBAP), in an electrochemical cell with a platinum working electrode. The reaction sequence to generate an excited state and light emission is:

where A and D could be the same species, e.g., an aromatic hydrocarbon such as rubrene. The term electrogenerated chemiluminescence (ECL) was coined to describe this class of reaction [1,2] and to distinguish it from other systems where light is emitted from an electrode in electrochemical cells.
As pointed out in earlier reviews [3,4], light can be emitted during an electrochemical reaction in a number of different ways, and reports concerning such emission date back at least to the 1880s. These types of reactions are briefly described below but are not considered within the class of ECL reactions and are not dealt with further in this monograph.
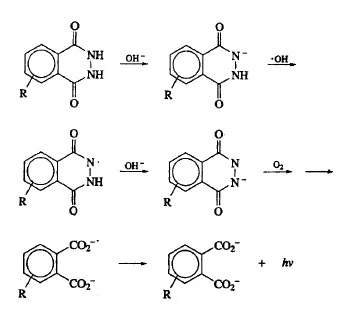
Scheme 1
A. Chemiluminescence Reactions
There are numerous chemical reactions where the decomposition of a species, often triggered by reaction with oxygen or peroxide, results in the production of excited states and emission [5]. The best known, e.g., those involving luminol, luciginen, and oxalate esters, generate intense light and are the basis for consumer products such as “light sticks.” Consider the luminol (5-amino-2,3-dihydro-1,4-phthalazinedione) chemiluminescent reaction (R = NH2) shown in Scheme 1. This chemiluminescent reaction is generally carried out in alkaline solutions by the addition of hydrogen peroxide in the presence of an ion such as Fe2+, which generates hydroxyl radical. The mechanism is complicated and leads to destruction of the luminol starting material, with the emitter, the excited phthalate, completely different than the starting material. One can generate chemiluminescence in an electrochemical cell, for example by generating peroxide by the reduction of oxygen or oxidizing luminol [6,7]. Although this reaction can be useful, for example in imaging reactions at electrodes [8], it is not the type of reaction considered in this volume. Perhaps it would be useful to classify these in a more general class as electrochemiluminescence.
B. Glow Discharge Electrolysis
If electrolysis is carried out with a small electrode, e.g. the anode, and very high current densities (and high voltages) are applied, the evolution of gas (e.g., oxygen at the anode) forms a gas layer that separates solution from the electrode. At higher applied voltages, breakdown occurs in the gas film, leading to a glow discharge and emission of light [9]. This phenomenon is closely related to gas discharge and also occurs when one of the electrodes in the cell is moved into the gas phase above the liquid [10]. The emission of light results from ion–radical and electron–radical reactions that have been widely studied in gas-phase chemiluminescence [5].
C. Electroluminescence
There are several mechanisms by which light is emitted in solids, usually semiconductors or insulators [11]. Dielectric breakdown, similar to the glow discharge phenomenon that occurs in gases, can produce light emission. This involves rather high electric fields. Another high-field form of luminescence in solids is related to cathodoluminesence, which occurs when cathode rays (beams of electrons) strike a phosphor, as in cathode ray tubes in oscilloscopes. In this case the electron beam behaves as an excitation source, like the ultraviolet excitation beam in fluorescence, and causes excitation via electronic transitions in the solid material. This mechanism, sometimes called acceleration–collision electroluminescence [11], occurs in many electroluminescence (EL) devices, such as those with ZnS, where electrons injected into the conduction band are accelerated by the applied electric field and cause excitation of activator centers produced by doping of the ZnS.
An EL process that is closer to ECL is one that involves electron–hole recombination in the solid following injection of carriers (sometimes called injection electroluminescence). This is the process responsible for light emission at suitable p-n junctions in light-emitting diodes (LEDs), for example, those of GaAs. This process can also occur at semiconductor electrodes in electrochemical cells. For example, when an electrode of ZnS doped with Mn immersed in a solution containing peroxydisulfate (S2O82–) passes cathodic current, fairly intense light emission centered at about 580 nm is observed [12]. This emission results from injection of electrons into the conduction band of the ZnS:Mn, as shown schematically in Figure 1. The passage of current leads to the reduction of the S2O82– and in turn the production of the strong oxidant, SO4−•. The latter can inject a hole into the valence band of the ZnS:Mn, resulting in electron–hole recombination and emission. This process is formally the same as the use of S2O82– as a coreactant in ECL systems as described below and in Chapter 5. This same EL process occurs with many semiconductor electrodes and also with thin oxide films on metals such as Ta and Al. These are considered briefly in Chapter 11.
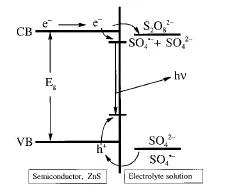
Figure 1 Electroluminescence of ZnS electrode immersed in a 0.2 M Na2SO4 solution containing 0.2 M ammonium peroxydisulfate. (See Ref. 12).
II. HISTORY OF ECL
A. Background
To understand how ECL experiments arose in the middle 1960s, it is useful to consider the scientific environment that existed at the time, especially with respect to the electrochemistry of organic compounds and to studies of radical ions. The state of organic electrochemistry in the 1950s was quite confused. The problem was that most studies were carried out in aqueous and partially aqueous solutions (e.g., mixtures of water with ethanol or dioxane to improve solubility) [13]. Water has the disadvantage of having a limited range of available potentials before it is oxidized to oxygen or reduced to hydrogen (i.e., it has a small potential window). Moreover, water shows reasonable acidic and basic properties so that proton transfer reactions are frequently coupled to electron transfers in this solvent. The result was that many of the electrode reactions showed waves resulting from the addition of two or more electrons. For example, the reduction of anthracene was described as a two-electron, two-proton reduction leading to 9,10-dihydroanthracene as the product. Moreover, many oxidation reactions of organic compounds were described in terms of adsorbed intermediates, with reactions like the Kolbe reaction being very popular [14]. Radical ions were not thought of as the usual intermediates, because the prevailing concepts of organic chemistry at the time were based largely on “pushing” electron pairs, in contrast to the currently accepted idea of electrode reactions generally proceeding in elementary steps involving single electron transfer.
The situation began to change with the introduction of the use of aprotic solven...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Preface
- Contributors
- 1 Introduction
- 2 Experimental Techniques of Electrogenerated Chemiluminescence
- 3 ECL Theory: Mass Transfer and Homogeneous Kinetics
- 4 Electron Transfer and Spin Up-Conversion Processes
- 5 Coreactants
- 6 Organic ECL Systems
- 7 Metal Chelate Systems
- 8 Clinical and Biological Applications of ECL
- 9 Analytical Applications: Flow Injection, Liquid Chromatography, and Capillary Electrophoresis
- 10 ECL Polymers and Devices
- 11 Miscellaneous Topics and Conclusions
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Electrogenerated Chemiluminescence by Allen J. Bard in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.