
- 552 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Separation Techniques in Clinical Chemistry
About this book
This reference examines innovations in separation science for improved sensitivity and cost-efficiency, increased speed, higher sample throughput and lower solvent consumption in the assessment, evaluation, and validation of emerging drug compounds. It investigates breakthroughs in sample pretreatment, HPLC, mass spectrometry, capillary electrophor
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Sample Pretreatment in Clinical Chemistry
I. N. Papadoyannis and V. F. Samanidou
Aristotle University of Thessaloniki, Thessaloniki, Greece
1 INTRODUCTION
The development of any analytical method includes a number of steps from sample collection to the final report of the results. Intermediate stages involve sample storage, sample preparation, matrix modification, isolation of ana-lytes, identification, and finally quantification. Among theses steps the most tedious and time-consuming is the preanalytical phase, known as sample pretreatment, which takes almost 60% of the time distribution of the whole procedure of analysis and determination. It is also the most error-prone part of the process, as it contributes 30% of the sources of errors, impacting on precision and accuracy of the overall analysis [1–3].
In clinical chemistry, sample preparation is crucial, as the determination and identification of endogenous compounds is important for the diagnosis and prevention of disorders. Analytical chemistry is continuously used in the research and development of new drugs, e.g., for pharmacokinetics, drug monitoring, metabolism studies, and toxicological studies.
The pretreatment of samples of biological origin is aimed mainly at the adequate reduction in matrix interference. The successful extraction of drugs from biological matrices such as urine, blood, saliva, and hair presents several challenges, as their composition is variable and complex, and the concentrations of analytes are very low. Biological matrices often contain proteins, salts, acids, bases, and various organic compounds with similar chemistries to the target analytes. Selective analysis of drugs from a variety of biological matrices can be performed by both traditional and modern techniques of sample pretreatment. The objectives of sample pretreatment are listed in Table 1. The term sample pretreatment may refer to various stages of the analysis procedure as shown in Fig. 1 [4–11].
Several sample pretreatment techniques are reported in the literature depending on the sample volume and required quantities for measurement. These techniques can also be classified in terms of sample nature, liquid or solid, as shown in Table 2 [10].
An ideal sample pretreatment technique should have the following characteristics:
Simplicity and rapidity
High extraction efficiency with quantitative and reproducible analyte recoveries
Specificity for the analytes
High sample throughput; fewer manipulation steps to minimize analyte losses
Amenability to automation
Use of the minimum amount of solvent, compatible with many analytical techniques
Low cost, regarding reagents and equipment [12]
Although there is no single ideal technique for sample preparation, there are several, each with its own advantages and disadvantages. The most commonly used techniques are discussed in the following paragraphs of this chapter with the emphasis on clinical samples. The analyst may choose the most appropriate technique, when developing a method depending on several parameters such as sample matrix, nature, physical and chemical properties of analytes, concentration, and analytical technique that is to be applied.
TABLE 1 Objective of Sample Preparation
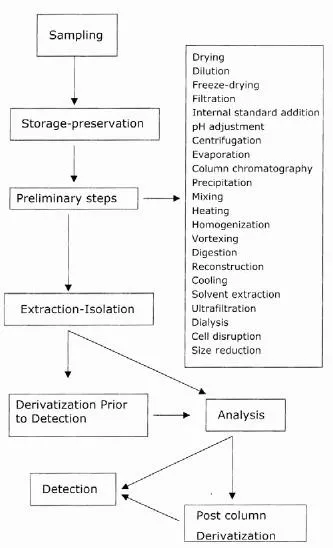
FIGURE 1 Stages of sample preparation. (From Refs. 9–11.)
As the biggest problem with sample preparation is time, an increasingly important consideration when developing techniques is the possibility of automating the entire analytical process. The benefits of automation, apart from the obvious economic ones, the reduced number of manual operations and amount of laboratory equipment required, include increased sample throughput and productivity per instrument and higher precision. Moreover, automation may improve contamination control in trace analysis, as handling of contagious or radioactive samples can be performed in closed analytical systems. Thus high-throughput automated analyzers are the work-horses in the central hospital laboratory [7,10].
TABLE 2 Typical Preparation Techniques for Liquid and Solid Samples
2 SAMPLES OF CLINICAL INTEREST
Clinical analysis deals mainly with the determination of drugs, metabolites, poisons, chemicals of environmental exposure, and endogenous substances in body fluids and tissues. Additionally, the quantitative and qualitative analysis of drugs and metabolites is applied to pharmacokinetic studies. Variables such as time to maximal concentration in plasma, clearance, and bioavail-ability have to be identified for the approval of a new drug. Therapeutic drug monitoring (TDM) may be a useful tool for the improvement of drug therapy. Drugs of abuse and illicit drugs are analyzed in clinical and forensic toxicology. Finally, as part of environmental medicine, a wide variety of chemicals such as dioxins are analyzed in human body fluids for the investigation of environmental and occupational exposure.
The nature and typical characteristics of the most common biological samples are discussed in this section.
2.1 Urine
This is one of the most commonly studied biological matrices for drug determination, and its analysis is of paramount importance in clinical chemistry, as it is relatively easy to collect. It can be used for drug screening, for forensic purposes, and to monitor workplace exposure to chemicals. It is a universal means of excretion of both parent drug compound and diagnostic metabolites. As a matrix, it has moderate complexity and a relatively high variability, and it typically contains both organic and inorganic constituents. Drug extraction efficiency can vary for several reasons such as variations in pH value and ionic strength and the presence of additional components [13,14].
2.2 Blood: Serum—Plasma—Whole Blood
Serum is the straw-colored liquid that separates from the clot that is formed in whole blood. Plasma is prepared from whole blood that has been treated with an anticlotting substance such as heparin. It is the supernatant that results when the cellular components of blood are removed by centrifugation. As matrices for drug analysis, the significant difference between the two is that serum does not contain fibrinogen and certain clotting factors (2.5–5% of proteins). Both serum and plasma are routinely used for drug analysis. A method developed for plasma can normally be applied to serum without modification.
Plasma samples contain significant amounts of salt and protein. Major constituents of normal human serum are albumins 35–45 mg/mL, globulins 30–35 mg/mL, lipids 4–7 mg/mL, salts 7 mg/mL, and carbohydrates 1.34–2.0 mg/mL. Drugs will bind to plasma proteins to varying degrees depending on their individual physicochemical properties. In general, acid and natural drugs bind primarily to albumin, and basic drugs primarily to a-acid glycoprotein. Although it is general practice to report total drug concentration (free plus protein-bound) in serum, there are isolation techniques, like solid-phase microextraction (SPME), that can measure the concentration of free drug, which is therapeutically relevant.
In most cases a method developed for the determination of drugs in plasma could also apply to the analysis of whole blood, which is particularly useful in postmortem analyses. The determination of blood constituents can be performed either by employing headspace analysis, as blood is considered a ‘‘dirty’’ sample, or by means of direct immersion extraction, e.g., a range of local anesthetics [13,14].
2.3 Saliva
The determination of drugs in saliva is convenient, as sampling is noninvasive. A sample is easy to collect, and quantitative measurements may reflect the nonpolar protein bound, i.e., the therapeutically relevant fraction of the drug in plasma. Compared to other biological samples, levels of protein and lipids are quite low, making it amenable to microextraction. Extraction efficiency from saliva may vary from 5 to 9% relative to 100% for extraction from pure water, owing to a combination of reduced mass transfer kinetics in the sample due to its viscous nature, and binding of the compounds to proteins present in the sample. By acidifying the samples with acetic acid, extraction efficiencies are improved as the samples are clarified, and proteinaceous material and cellular debris are precipitated and removed prior to extraction [13,14].
2.4 Tissue
Tissue samples are treated as solid samples and can originate from liver, myocardium, muscle, kidney cortex, cerebellum, and brain stem. Tissue samples are treated by an extraction technique, or headspace sampling is applied for volatile compounds.
2.5 Hair
Hair is a popular target for drug analysis as collection is relatively non-invasive; therefore its analysis can be used for forensic purposes, and to monitor drug compliance and abuse. As with biological fluids, drugs and their metabolites are expressed in hair. Measurements along a strand of hair can provide a record of drug usage or exposure. Before analysis the hair matrix must be either digested enzymatically (e.g., with a protease) or more usually with strong alkali, e.g., 1 M NaOH. Though it suffers from variability in drug concentration by hair type, due to drug affinity variations, an additional advantage is that it is a fairly nonpolar matrix, so it tends to absorb parent drug molecules, which are typically less polar than metabolites. Because of this, it is ideal for extraction of analytes to nonpolar extraction phases, especially when the parent drug is extensively metabolized and often non-detectable in other tissues [13–15].
2.6 Human Milk
The main constituents of human milk are water (88%), proteins (3%), lipids (3%), and carbohydrate in the form of lactose (6%). The lipids are in the form of fat droplets suspended in the aqueous matrix. The nonwater constituents are present in different physical forms: dissolved (lactose), colloidally dispersed (protein), and in water (lipids). Human milk can serve as a means of exposure of a newborn to compounds the mother has been previously exposed to and provides a relatively convenient means of biomonitoring for toxicant exposure. It is quite constant in composition from the third week on, while collostrum from early lactation, differs sign...
Table of contents
- COVER PAGE
- TITLE PAGE
- COPYRIGHT PAGE
- PREFACE
- CONTRIBUTORS
- 1. SAMPLE PRETREATMENT IN CLINICAL CHEMISTRY
- 2. SEPARATION SCIENCE IN THERAPEUTIC DRUG MONITORING
- 3. THE IMPACT OF CHIRALITY IN PHARMACOKINETICS AND THERAPEUTIC DRUG MONITORING
- 4. HPLC IN BIOAVAILABILITY EXAMINATION
- 5. VALIDATION OF HPLC ANALYSES IN THERAPEUTIC DRUG MONITORING
- 6. ANALYSIS OF ILLICIT DRUGS WITH CHROMATOGRAPHIC METHODS
- 7. BIOGENIC AMINE NEUROTRANSMITTERS: THEIR IMPORTANCE AND MEASUREMENT IN HUMAN TISSUE AND BODY FLUIDS
- 8. CLINICAL APPLICATIONS OF AFFINITY CHROMATOGRAPHY
- 9. IMMUNOAFFINITY CHROMATOGRAPHY IN CLINICAL ANALYSIS
- 10. ELECTROSPRAY TANDEM MASS SPECTROMETRY IN THE BIOCHEMICAL GENETICS LABORATORY
- 11. APPLICATION OF THIN-LAYER CHROMATOGRAPHY IN CLINICAL CHEMISTRY
- 12. APPLICATIONS OF CAPILLARY ELECTROPHORESIS IN CLINICAL ANALYSIS OF DRUGS
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Separation Techniques in Clinical Chemistry by Hassan Y. Aboul-Enein in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.