
- 544 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The field of bioseparation, and biochromatography in particular, is advancing very rapidly as our knowledge of the properties of molecules and atomic forces increases. This volume covers the basic principles of biochromatography in detail. It assesses different techniques and includes a large number of applications, providing the reader with a mult
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
MedicineSubtopic
Science Research & MethodologyChapter 1
Upstream and downstream steps in biochromatography
Mookambeswaran A.Vijayalakshmi
Introduction
The “Biochromatography” or “chromatography of biological molecules” is a multidisciplinary field, involving the expertise development in:
- Polymer chemistry, for the development of column packing materials/adsorbents with different composition, different derivatization and different particle sizes etc.
- Mechanical designing of the columns with fluid mechanics knowledge.
- Development of software for data handling, operation controls and even simulations.
- Detection systems with optics, lasers, amperometrics etc.
- Sophisticated physico-chemical analytical methods on-line or off-line such as ESI-MS.
The striking development of highly efficient and sophisticated microanalytical and preparative chromatography has led to tremendous progress in the study of proteins and peptides. Nevertheless, optimisation of upstream and downstream operations, in chromatography steps, such as finding the right conditions of extraction, sample preparation and suitable methods for monitoring the protein and biological activities remain crucial. Several manuals are available which cover the detailed operations of protein separation and purification covering relevant details of the extraction methods (Scopes, 1987; Ladish et al., 1990; Janson and Ryden, 1989; Methods in Enzymology, 22, 34 and 204).
We can divide protein separation and purification into four broad steps: extraction; fractional precipitation; purification and final polishing (Fig. 1.1). Before these four steps the source identification and choice of raw material for the targetted protein is an important issue which in turn, will determine/orientate the choice of extraction methods.
Choice of sources of raw materials
In the case, particularly, of biochromatographic operations of proteins, the following questions have to be answered before designing any experimental set up (Fig. 1.2). What is the final targetted use of the protein? Is the protein for purification meant for basic studies on the protein, such as crystal structure or is it meant for large scale production to be used in agro food industry or pharmaceutical industry? If the final use is pharmaceutical, then one has to take into consideration the high demand on the purity of the final product: 99.9–99.99%. In terms of choice of raw material, these questions have a high relevance. If we use animal sources e.g. albumin or IgG from animal/human sera, we have to seriously consider alternative sources to avoid the potential HIV, Hepatitis B, etc. contamination, from the raw material.
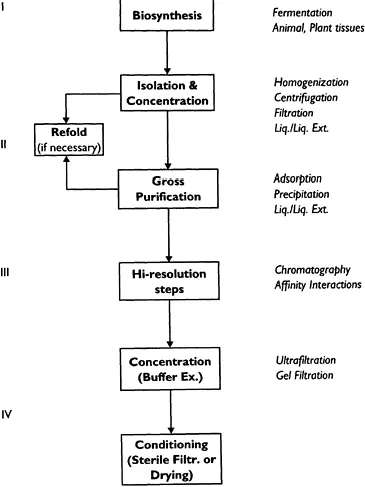
Figure 1.1 General purification scheme with the four major steps, in downstream processing.
These alternative sources are mainly based on recombinant technology; human/animal proteins are expressed in micro-organisms or humanised cells or plant cells or whole plants, seeds, fruits etc. The use of the latter sources, also termed as Molecular Farming, is more recent and is quite promising in terms of cost effective and facile production of the raw material. But, here the purification, particularly the extraction steps prior to the chromatographic steps needs special care, both in terms of the enormous quantity of the raw material to be treated (leaves, seeds, fruits etc.) and also the composition of buffers (e.g. addition of polyvinylpyrolidone) for preventing the complexation of the target proteins with polyphenols.
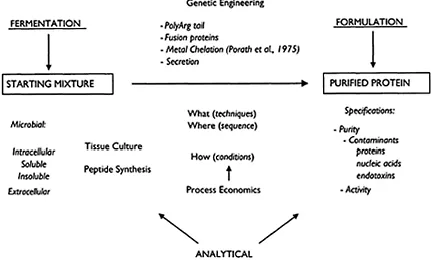
Figure 1.2 The multifaceted nature of protein purification process development. (From HO 1990 with permission.)
Extraction methods
The extraction step is meant for obtaining the target molecule (protein) in solution. It is obvious that certain raw materials of biological origin constitute an almost clear solution of the protein. Some of the examples are: blood sera, urine, milk, extracellular protein in the animal, bacterial, yeast, mammalian and plant cells culture media. The main upstream step in these cases is just the cell separation and concentration step by membrane (ultrafiltration) processes or by precipitation techniques.
In the case of insoluble raw materials such as animal or vegetal tissues and in the case of intracellular proteins produced in the cell culture systems, not only adequate extraction methods should be used to obtain the protein in its soluble form, but also specific additives such as pro tease inhibitors, reducing agents, polyphenol blockers (e.g. polyvinylpyrolidone) should be added to the extraction media.
A classification of extraction methods and their applicability to different raw materialsis described in Table 1.1.
The use of detergents in the extraction step needs some special comments. The addition of detergents is used mainly for the extraction of membrane bound proteins, by reducing the hydrophobic interactions involved in the protein binding to the membranes. Some chaotopic agents can replace detergents for the same purpose. Some detergents may denature the proteins; hence attention has to be paid both for the choice of the detergents for extraction, their concentration and also whether to keep the presence of detergent in all the steps including the final chromatographic steps. This last point evokes the question of compatibility of the specific detergent added to the buffer and the chromatographic adsorption media chosen. Non-ionic detergents such as Triton x-100 are usually more compatible with the different affinity adsorbants than the ionic detergents such as Sodium Dodecyl Sulfate (SDS). Moreover SDS can denature the protein and interfere with its biological activity. The ionic, non-ionic and zwitterionic nature of the specific detergent may have an impact both on protein surface as well as on the chromatographic adsorbent properties, thus influencing both adsorption and the elution of the protein in the chromatographic steps. Thus a fairly good knowledge of the chemical structure of the detergent to be chosen is very useful. The reader can get more information on the detergents and their physicochemical properties, in Hjelmeland’s chapter in Methods in Enzymology vol. 124, 1986.
Table 1.1 Initial fractionation (corresponding to step 1 of Fig. 1.1)
The optimum concentration of the detergent to be used, should invariably be below the critical micelle concentration, in order to avoid the protein being entrapped into the micelle and thus not able to interact with chromatographic adsorbent surfaces. However, in the electrokinetic chromatographic mode, the protein-detergent micelles are exploited for separating/studying the proteins as a function of their size (Landers, 1993).
The other additives to be used, including the buffers, during the extraction step and up to the chromatographic steps are enumerated in Table 1.2.
Table 1.2 Extraction medium
Fractionation techniques
This step is intended mainly for reducing the volume of the solution to be handled in further steps. It also reduces the total number of components present in the medium. The fractionation methods are mainly based on precipitation of the target protein by decreasing its solubility by modifying the salt concentration (“salting in” and “salting out” approaches); or by the addition of organic solvents or organic polymers. Temperature and/or pH variations leading to targetted denaturation of the contaminating proteins and precipitation of the desired protein may also be used as a fractionation step.
In the “salting out” approach the property of a particular salt (e.g. (NH4)2SO4) as an efficient precipitation agent is deduced from the so called Hofmeister series anions:
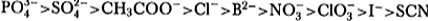
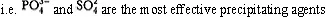
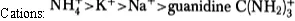
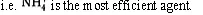
Hence it follows from the above that (NH4)2 SO4 is a very good precipitation agent. Moreover, this salt ensures minimisation of any denaturation as it stabilises the native conformation of the protein to be precipitated.
Organic solvents promote the precipitation of proteins by disturbing the organised water molecules around the protein. Even though, it may, at first sight look like organic solvents may denature the proteins, two organic solvents namely, ethanol and acetone have been very successfully used for the selective precipitation of serum proteins, enzymes or hormones.
The ethanol precipitation of serum proteins known as the “COHN fractionation” method introduced in 1946 (Cohn, 1946) is used even today at all levels, may be with some modification (Taylor, 1956). This method, by a proper control of pH and temperature, enables one to prepare fractions particularly enriched in albumin, β globulins and γ globulins. In addition, the use of ethanol and subzero temperatures ensures certain safety against microbial and other contamination during the process.
The organic polymers such as polyethylene glycol (PEG) in the molecular weight range of 6000 to 20000 are able to precipitate the proteins by sequestrating the water molecules in a rather similar way to the organic solvents (Curling, 1980). PEG is the most widely used polymer due to its inocuity, non toxicity, low immunogeneicity etc.
In some cases inorganic polymers are used to concentrate the protein solutions. Here the phenomenon exploited is not a precipitation, but a physical adsorption. A case in study is the use of silica or cesium, Kieselger etc. to concentrate proteins from culture broth or even from urine. The adsorbed proteins are then stripped off using minimum quantity of buffers to be used in further s...
Table of contents
- Cover Page
- Title Page
- Copyright Page
- Contributors
- Acknowledgements
- Preface
- Foreword
- Chapter 1: Upstream and Downstream Steps in Biochromatography
- Chapter 2: Gel Filtration
- Chapter 3: Ion Exchange Interaction Biochromatography
- Chapter 4: Hydrophobic (Interaction) Chromatography of Proteins
- Chapter 5: Conformational Behaviour of Polypeptides and Proteins in Reversed Phase and Lipophilic Environments
- Chapter 6: Affinity Chromatography
- Chapter 7: Dye Ligand Affinity Chromatography
- Chapter 8: Immobilized Synthetic Dyes in Affinity Chromatography
- Chapter 9: Immobilized Histidine Ligand Affinity Chromatography
- Chapter 10: Immobilized Metal-ion Affinity Chromatography: From Phenomenological Hallmarks to Structure-based Molecular Insights
- Chapter 11: Thiophilic Interaction Chromatography
- Chapter 12: Miscellaneous Methods in Affinity Chromatography Part 1: Boronic Acids as Selective Ligands for Affinity Chromatography
- Chapter 12: Miscellaneous Methods in Affinity Chromatography Part 2: Shielded Affinity Chromatography in Packed Bed and Expanded Bed Mode
- Chapter 13: Glycobiology and Biochromatography
- Chapter 14: Capillary Electrokinetic Chromatography
- Chapter 15: Imprinted Polymers as Tailor-made Stationary Phases for Affinity Separation
- Chapter 16: Computer-aided simulation of biochromatography
- Chapter 17: Industrial Biochromatography: Engineering Aspects
- Chapter 18: Validation Aspects in Biochromatography
- Chapter 19: Biochromatography and Biomedical Applications
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Biochromatography by M. A. Vijayalakshmi in PDF and/or ePUB format, as well as other popular books in Medicine & Science Research & Methodology. We have over one million books available in our catalogue for you to explore.