
- 720 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Nano-Surface Chemistry
About this book
Containing more than 2600 references and over 550 equations, drawings, tables, photographs, and micrographs, This book describes hierarchical assemblies in biology and biological processes that occur at the nanoscale across membranes and at interfaces. It covers recurrent themes in nanocolloid science, including self-assembly, construction of supra
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Molecular Architectures at Solid–Liquid Interfaces Studied by Surface Forces Measurement
KAZUE KURIHARA Tohoku University, Sendai, Japan
I. INTRODUCTION
Molecular and surface interactions are ubiquitous in molecular science, including biology. Surface forces measurement and atomic force microscopy (AFM) have made it possible to directly measure, with high sensitivity, molecular and surface interactions in liquids as a function of the surface separation. Naturally, they have become powerful tools for studying the origins of forces (van der Waals, electrostatic, steric, etc.) operating between molecules and/or surfaces of interest [1–4]. They also offer a unique, novel surface characterization method that “monitors surface properties changing from the surface to the bulk (depth profiles)” and provides new insights into surface phenomena. This method is direct and simple. It is difficult to obtain a similar depth profile by other methods; x-ray and neutron scattering measurements can provide similar information but require extensive instrumentation and appropriate analytical models [4].
Molecular architectures are self-organized polymolecular systems where molecular interactions play important roles [5]. They exhibit specific and unique functions that could not be afforded by single molecules. Molecular architecture chemistry beyond molecules is not only gaining a central position in chemistry but becoming an important interdisciplinary field of science. Investigations of molecular architectures by surface forces measurement is important for the following reasons.
- It is essential to elucidate intermolecular interactions involved in self-organization, whose significance is not limited to material science but extends to the ingenuity of biological systems [5].
- The importance of surface characterization in molecular architecture chemistry and engineering is obvious. Solid surfaces are becoming essential building blocks for constructing molecular architectures, as demonstrated in self-assembled monolayer formation [6] and alternate layer-by-layer adsorption [7]. Surface-induced structuring of liquids is also well-known [8,9], which has implications for micro-and nano-technologies (i.e., liquid crystal displays and micromachines). The virtue of the force measurement has been demonstrated, for example, in our report on novel molecular architectures (alcohol clusters) at solid–liquid interfaces [10].
- Two-dimensionally organized molecular architectures can be used to simplify the complexities of three-dimensional solutions and allow surface forces measurement. By employing this approach, we can study complex systems such as polypeptides and polyelectrolytes in solutions. For example, it is possible to obtain essential information such as the length and the compressibility of these polymers in solutions by systematically varying their chemical structures and the solution conditions [11].
Earlier studies of surface forces measurement were concerned mainly with surface interactions determining the colloidal stability, including surfactant assemblies. It has been demonstrated, however, that a “force–distance” curve can provide much richer information on surface molecules; thus it should be utilized for studying a wider range of phenomena [12]. Practically, the preparation of well-defined surfaces, mostly modified by two-dimensional organized molecules, and the characterization of the surfaces by complementary techniques are keys to this approach. A similar concept is “force spectroscopy” [13], coined to address force as a new parameter for monitoring the properties of materials. A major interest in force spectroscopy is the single molecular measurement generally employing an atomic force microscope. This measurement treats relatively strong forces, such as adhesion, and discusses the binding of biotin-streptavidin [14] and complementary strands of DNA [15] as well as the unfolding and folding of proteins [16]. On the other hand, the forces measurement of two-dimensionally organized molecules has advantages complementary to those of single molecule force spectroscopy. It can monitor many molecules at the same time and thus is better suited for studying long-range weaker forces. The measurement should bear a close relevance to real systems that consist of many molecules, because interactions between multiple molecules and/or macroscopic surfaces in solvents may exhibit characteristics different from those between single molecules.
The aim of this review is to demonstrate the potential of surface forces measurement as a novel means for investigating surfaces and complex soft systems by describing our recent studies, which include cluster formation of alcohol, polyion adsorption, and polyelectrolyte brushes.
II. SURFACE FORCES MEASUREMENT
Surface forces measurement directly determines interaction forces between two surfaces as a function of the surface separation (D) using a simple spring balance. Instruments employed are a surface forces apparatus (SFA), developed by Israelachivili and Tabor [17], and a colloidal probe atomic force microscope introduced by Ducker et al. [18] (Fig. 1). The former utilizes crossed cylinder geometry, and the latter uses the sphere-plate geometry. For both geometries, the measured force (F) normalized by the mean radius (R) of cylinders or a sphere, F/R, is known to be proportional to the interaction energy, Gƒ, between flat plates (Derjaguin approximation),

This enables us to quantitatively evaluate the measured forces, e.g., by comparing them with a theoretical model.
Sample surfaces are atomically smooth surfaces of cleaved mica sheets for SFA, and various colloidal spheres and plates for a colloidal probe AFM. These surfaces can be modified using various chemical modification techniques, such as Langmuir–Blodgett (LB) deposition [12,19] and silanization reactions [20,21]. For more detailed information, see the original papers and references texts.
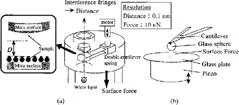
FIG. 1 Schematic drawings of (a) the surface forces apparatus and (b) the colloidal probe atomic force microscope.
III. ALCOHOL CLUSTER FORMATION ON SILICA SURFACES IN CYCLOHEXANE
Surface forces measurement is a unique tool for surface characterization. It can directly monitor the distance (D) dependence of surface properties, which is difficult to obtain by other techniques. One of the simplest examples is the case of the electric double-layer force. The repulsion observed between charged surfaces describes the counterion distribution in the vicinity of surfaces and is known as the electric double-layer force (repulsion). In a similar manner, we should be able to study various, more complex surface phenomena and obtain new insight into them. Indeed, based on observation by surface forces measurement and Fourier transform infrared (FTIR) spectroscopy, we have found the formation of a novel molecular architecture, an alcohol macrocluster, at the solid–liquid interface.
Adsorption phenomena from solutions onto solid surfaces have been one of the important subjects in colloid and surface chemistry. Sophisticated application of adsorption has been demonstrated recently in the formation of self-assembling monolayers and multilayers on various substrates [4,7]. However, only a limited number of researchers have been devoted to the study of adsorption in binary liquid systems. The adsorption isotherm and colloidal stability measurement have been the main tools for these studies. The molecular level of characterization is needed to elucidate the phenomenon. We have employed the combination of surface forces measurement and Fourier transform infrared spectroscopy in attenuated total reflection (FTIR-ATR) to study the preferential (selective) adsorption of alcohol (methanol, ethanol, and propanol) onto glass surfaces from their binary mixtures with cyclohexane. Our studies have demonstrated the cluster formation of alcohol adsorbed on the surfaces and the long-range attraction associated with such adsorption. We may call these clusters macroclusters, because the thickness of the adsorbed alcohol layer is about 15 nm, which is quite large compared to the size of the alcohol. The following describes the results for the ethanol–cycohexane mixtures [10].
Typical forces profiles measured between glass surfaces in ethanol–cyclohexane mixtures are shown in Fig. 2. Colloidal probe atomic force microscopy has been employed. In pure cyclohexane, the observed force agrees well with the conventional van der Waals attraction calculated with the nonretarded Hamaker constant for glass/cyclohexane/glass, 3.1 × 10-21 J. At an ethanol concentration of 0.1 mol%, the interaction changes remarkably: The long-range attraction appears at a distance of 35 nm, shows a maximum around 10 nm, and turns into repulsion at distances shorter than 5 nm. The pull-off force of the contacting surfaces is 140 ± 19 mN/m, which is much higher than that in pure cyclohexane, 10 ± 7 mN/m. Similar force profiles have been obtained on increasing the ethanol concentration to 0.4 mol%. A further increase in the concentration results in a decrease in the long-range attraction. At an ethanol concentration of 1.4 mol%, the interaction becomes identical to that in pure cyclohexane. When the ethanol concentration is increased, the range where the long-range attraction extends changes in parallel to the value of the pulloff force, indicating that both forces are associated with the identical phenomenon, most likely the adsorption of ethanol. Separation force profiles after the surfaces are in contact shows the presence of a concentrated ethanol layer near and on the surfaces (see Ref. 10a)...
Table of contents
- COVER PAGE
- TITLE PAGE
- COPYRIGHT PAGE
- PREFACE
- CONTRIBUTORS
- INTRODUCTION
- 1. MOLECULAR ARCHITECTURES AT SOLID–LIQUID INTERFACES STUDIED BY SURFACE FORCES MEASUREMENT
- 2. ADHESION ON THE NANOSCALE
- 3. LANGMUIR MONOLAYERS: FUNDAMENTALS AND RELEVANCE TO NANOTECHNOLOGY
- 4. SUPRAMOLECULAR ORGANIC LAYER ENGINEERING FOR INDUSTRIAL NANOTECHNOLOGY
- 5. MONO-AND MULTILAYERS OF SPHERICAL POLYMER PARTICLES PREPARED BY LANGMUIR–BLODGETT AND SELF-ASSEMBLY TECHNIQUES
- 6. STUDIES OF WETTING AND CAPILLARY PHENOMENA AT NANOMETER SCALE WITH SCANNING POLARIZATION FORCE MICROSCOPY
- 7. NANOMETRIC SOLID DEFORMATION OF SOFT MATERIALS IN CAPILLARY PHENOMENA
- 8. TWO-DIMENSIONAL AND THREE-DIMENSIONAL SUPERLATTICES: SYNTHESES AND COLLECTIVE PHYSICAL PROPERTIES
- 9. MOLECULAR NANOTECHNOLOGY AND NANOBIOTECHNOLOGY WITH TWO-DIMENSIONAL PROTEIN CRYSTALS (S-LAYERS)
- 10. DNA AS A MATERIAL FOR NANOBIOTECHNOLOGY
- 11. SELF-ASSEMBLED DNA/POLYMER COMPLEXES
- 12. SUPRAMOLECULAR ASSEMBLIES MADE OF BIOLOGICAL MACROMOLECULES
- 13. REVERSED MICELLES AS NANOMETER-SIZE SOLVENT MEDIA
- 14. ENGINEERING OF CORE-SHELL PARTICLES AND HOLLOW CAPSULES
- 15. ELECTRO-TRANSPORT IN HYDROPHILIC NANOSTRUCTURED MATERIALS
- 16. ELECTROLYTES IN NANOSTRUCTURES
- 17. POLYMER–CLAY NANOCOMPOSITES: SYNTHESIS AND PROPERTIES
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Nano-Surface Chemistry by Morton Rosoff in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.