![]()
Part I
Attention and Eye Movements
![]()
Chapter 1
The Neural Control of Visually Guided Eye Movements
Peter H. Schiller
Massachusetts Institute of Technology
Along with the eyes, Nature has created a system to move them about efficiently. The eyes of many species have become specialized in that they contain a small central region in the retina, the fovea, where the photoreceptors are tightly packed which consequently yields high acuity perception. Therefore, to be able to analyze an object in the visual scene in fine detail, the center of gaze has to be directed to it. In addition, when either the object or the person is in motion, it is desirable to maintain the center of gaze on the object. These requirements have produced two distinct systems of conjugate eye movements: the saccadic and the smooth pursuit. The function of the saccadic system is to acquire visual objects for central viewing; the function of the smooth pursuit system is to maintain objects on the fovea while either the object or the person is in motion.
Our eyes are on the move most of the time during our waking hours. We make about 3 saccadic eye movements per second, some 170,000 a day and about 5 billion in an average life time. During the intervening fixations, each of which lasts 200–500 ms, the eyes are stationary in the orbit only when neither the head nor the object viewed is in motion. If there is motion, the object remains on the fovea by virtue of the fact that the eyes engage in smooth-pursuit tracking.
The neural systems involved in the control of visually guided eye movements, the topic of this presentation, are numerous and complex yet are tremendously robust. Seldom does one hear about individuals complaining at the end of the day of having made those 170,000 saccades and endless pursuit eye movements.
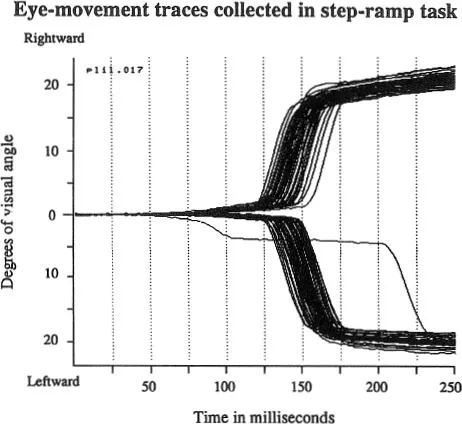
That these two types of eye movements, the saccadic and the smooth pursuit, are governed at higher levels by different neural systems has been known for a long time. When the velocity of an object to be tracked is gradually increased, a sudden break in performance occurs when tracking breaks down; the eye can no longer keep up with the moving object. When this happens, the saccadic system kicks in and moves the eyes to catch up with the object. Thus there is a clear velocity discontinuum between tracking and saccadic eye movements. The two systems also have dramatically different latency responses for the initiation of smooth pursuit and saccadic eye movements. This has first been shown by Rashbass (1961) who used what is now called a step-ramp paradigm. Following fixation of a spot on a homogeneous background, it is turned off and at the same time another spot appears somewhere in the periphery, which is then ramped at various velocities. The task of the subject is to make a saccade to the target and to track it. An example of this is shown in Fig. 1.1. The data in this case were collected from a monkey (Schiller & Logothetis, 1987). Examination of the eye traces shows something quite remarkable: The eyes begin to track the peripheral spot with a latency of 75 to 100 ms in this case, and do so before the saccade is initiated to it with a latency of 125 to 150 ms. In fact, it has been shown, that when a large portion of the visual field is set in motion, pursuit movements can be initiated in less time than 50 ms provided the stimuli have high contrast (Miles, Kawano, & Optican, 1986). High contrast assures rapid conduction velocities through the retina.
Fig. 1.1. Horizontal eye-movement traces obtained while a monkey performs on a step-ramp task. Following fixation of a central spot it is doused; at the same time a similar spot appears either to the right or the left of fixation at an 18° eccentricity and is moved peripherally along the horizontal axis at 20 deg/sec. Eye-movement trace collection began when the target was turned on in the periphery. The shorter latencies involved in activating the pursuit system are made evident by the fact that pursuit eye movements for the moving target actually begin before the monkey acquires it for foveal viewing with a saccade. Pursuit eye movements in this situation begin between 75 and 100 ms, whereas saccades are initiated between 125 and 150 ms. Adapted from Schiller and Logothetis (1987).
These observations have established, therefore, at the behavioral level, that there are different neural mechanisms involved in the control of saccadic and pursuit eye movements. In what follows I first discuss the various neural systems of saccadic eye-movement generation. Both the sensory and motor aspects of eye-movement production are considered. In the last section we take a brief look at the neural systems involved in pursuit eye movement.
Brainstem Control of Eye Movements
Each eye is moved around in the orbit using six extraocular muscles. Four of these are the recti muscles, the medial, lateral, superior, and inferior. Each opponent pair may be thought of as moving the eyes along two prime axes, the horizontal and the vertical. Diagonal eye movements are brought about by the combined action of the four recti muscles. The remaining two muscles, the superior and inferior obliques, participate mostly in inducing rotatory motion, the kind of motion that comes into play when the head is tilted. One of the important functions of the oblique muscles is to counter rotate the eyes so as to keep them stable with respect to the world.
The eye and its musculature have several features that make them the delight of engineers. The eye is a nearly perfectly balanced ball that is nicely viscous damped in its orbit. Unlike other muscle systems, it was not necessary to design the extraocular muscles to carry loads. The fibers of each muscle are not segmented: they run the entire length of the muscle. These facts make the analysis of eye motion readily amenable to study.
Three sets of cranial nuclei contain the neurons that innervate the six extraocular muscles of each eye through the third, fourth, and sixth cranial nerves: the oculomotor nuclei whose neurons innervate all the muscles except for the lateral rectus and the superior oblique, the trochlear nucleus whose neurons innervate the superior oblique, and the abducens nucleus whose neurons innervate the lateral rectus.
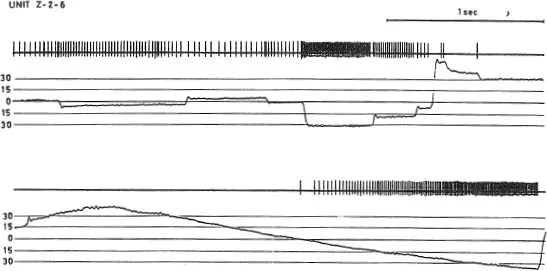
Figure 1.2 shows the response properties of a single cell in the oculomotor nucleus whose axon innervates the inferior rectus (Schiller, 1970). Shown are the action potentials for the cell over time and the monkey’s eye movements in the vertical plane. The upper set of traces were collected while the monkey looked around in the laboratory with his head restrained. Under such conditions the animal made saccadic eye movements with intervening fixations. The lower set of traces were collected while an object was moved downward in front of the monkey.
Fig. 1.2. Action potentials obtained from a single cell in the oculomotor nucleus that innervates the inferior rectus muscle. The activity of the neuron is shown along with vertical eye-movement traces. The upper set of records show neuronal activity while spontaneous eye movements are made and consist of saccadic eye movements with intervening fixations. The lower set of traces show neuronal activity during smooth-pursuit eye movement obtained by moving an object downward in front of the monkey. The rate of maintained activity of the neuron is linearly proportional to the angular displacement of the eye. Saccadic eye movements are associated with high-frequency bursts, the durations of which are proportional to saccade size. From Schiller (1970).
I emphasize three points about this figure. The first is that the rate of maintained activity exhibited by this neuron is proportional to the degree of downward deviation of the eye in orbit. The higher the activity the more acetylcholine is released at the terminals and consequently the more the inferior rectus contracts. It has been shown that there is a linear relationship between the degree of angular deviation of the eye and the rate of activity in neurons that form the final common path to the eye muscles. The second point is that the neuron discharges with a high frequency burst during the execution of downward saccadic eye movements; the size of the saccade is proportional to the duration of this high frequency burst. Upward saccades seen in the figure are associated with a pause of activity during which it is safe to assume that the neurons innervating the superior rectus discharge with high frequencies. The third point is that the neuron discharges in association with pursuit eye movements in a similar proportional fashion as was revealed when the monkey was fixating various objects in the stationary visual scene. This can be seen in the lower set of traces of Fig. 1.2.
I should note here one more interesting fact about the records shown in Fig. 1.2. Immediately after the execution of a saccadic eye movement brought about by a high-frequency neuronal burst, only a minimal overshoot can be seen. This is not accomplished by some sort of counteractivity in neurons innervating the antagonist muscle. If that were the case one would see in this record a brief burst immediately after the completion of an upward saccade. The remarkable ability of the eye to stop on a dime, so to speak, seems to be due simply to the excellent viscous damping achieved in tenon’s capsule within which the eye resides.
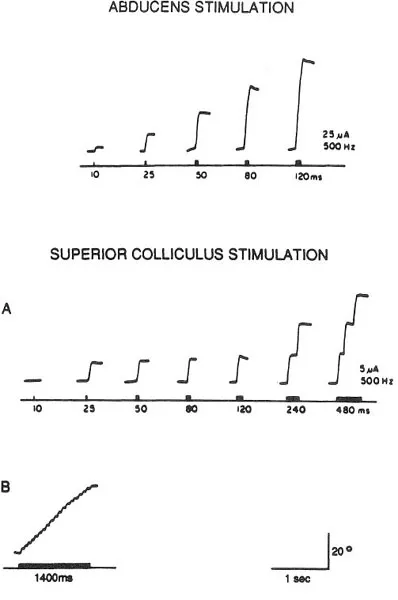
Eye movements can be artificially induced by electrically stimulating many different sites in the brain. The upper portion of Fig. 1.3 shows what happens when the abducens nucleus is stimulated electrically through a microelectrode. As the duration of the high-frequency burst is increased, the size of the saccade produced gets progressively larger as might be expected on the basis of what I had just described for natural neuronal discharges.
Fig. 1.3. The effects of electrically stimulating the abducens nucleus and the superior colliculus. Stimulation frequency is held constant at 500 Hz while burst duration is systematically varied. Stimulation of the abducens nucleus shows increasing saccade size as a function of increasing burst duration. By contrast, stimulation of the superior colliculus at any given site always produces the same direction and amplitude saccade. For long duration bursts staircases of saccades are elicited in the superior colliculus; the size of each saccade remains of the same amplitude and direction. Adapted from Schiller and Stryker (1972).
On the basis of these observations it appears that at the level of the oculomotor complex in the brainstem, where the neurons reside whose axons innervate the extraocular muscles, the saccadic and smooth-pursuit eye movements are executed by the same set of neurons. The coding operation seen here may be termed a rate/duration code: the higher the maintained rate, the greater the angular deviation of the eye in orbit; the longer the duration of the high frequency burst seen in these neurons, the larger the saccade produced (Robinson, 1975; Schiller, 1970).
Right above the nuclei innervating the extraocular muscles there is a complement of neurons in the brain stem in which the various components of the neuronal responses associated with eye movements can be seen separately. Several classes of neurons have been identified (Fuchs, Kaneko, & Scudder, 1985). These include the following types: burst neurons that discharge in high-frequency bursts during saccadic eye movements but otherwise remain silent, omnipause neurons that fire at a constant rate but pause whenever a saccade is made, and tonic neurons whose discharge rate is proportional to angular deviation of the eye in orbit but do not have bursts or pauses associated with saccadic eye movements. It is assumed that the activity of these and several other classes of neurons drives the cells in the oculomotor, trochlear, and abducens nuclei to produce the desired saccadic and smooth pursuit eye movements.
The Superior Colliculus and Saccadic Eye Movements
In considering the role of the superior colliculus in eye-movement control it should first be pointed out that this structure is one that has undergone tremendous changes in the course of evolution. In more primitive animals that have little forebrain, such as toads and fish, this structure, which in these animals is called the optic tectum, is the major site of visual information processing and also participates in converting visual signals into motor outputs. Consequently, ablation of the optic tectum renders these animals virtually blind and incapable of the execution of visually triggered motor commands (Schiller, 1984).
In mammals, and particularly in primates that have a greatly expanded neocortex, visual analysis has been relegated largely to the geniculo-striate system and associated higher cortical areas. The superior colliculus, residing on the roof of the midbrain, has taken on a much more modest function which appears to involve predominantly saccadic eye-movement control (Schiller, 1984; Sparks, 1986; Wurtz & Albano, 1980). Stained coronal sections of this area reveal seven major layers. For the sake of simplicity we shall divide these into just upper and lower layers. The upper layers receive input predominantly from the retina and the occipital cortex. Single cells here respond vigorously to visual stimuli, but their receptive field properties, unlike those in the cortex, are not particularly interesting. They prefer small stimuli but are insensitive to differences in shape, orientation, and color, and most lack directional selectivity to the movement of stimuli. It is noteworthy, however, that the visual field is laid out in a neat topographic order in the colliculus, with its anterior portion representing the fovea, its posterior portion the periphery, its medial aspect the upper and its later aspect the lower visual field. In each colliculus the contralateral half of the visual field is represented.
In the deeper layers of the colliculus there is also an orderly arrangement that has to do with the coding of eye movements. To understand it, let us first consider Fig. 1.3 (Schiller & Stryker, 1972). In the lower portions of this figure eye-movement records are shown that were obtained when the colliculus was electrically stimulated. Such stimulation produces effects quite different from those obtained by abducens stimulation that is shown on the top of the figure. In the colliculus saccades of certain amplitudes and directions are elicited whose parameters are largely unaffected by the duration of the stimulation burst delivered. However, at long durations a staircase of saccades can be elicited where each ballistic eye movement has pretty much the same amplitude and direction. What determines the size and direction of each saccade is where in the colliculus one stimulates (Robinson, 1972; Schiller & Stryker, 1972). The lowest portion in the figure shows a staircase of tiny saccades that were obtained when the electrode was placed in the anterior portion of the structure. Thus stimulation of the anterior colliculus produces small saccades and stimulation of the posterior colliculus produces large ones. Stimulation of the medial portions of the colliculus produces upward and of the lateral portions of the colliculus downward saccades.
Systematic mapping of the receptive fields and the motor responses in the colliculus reveals a neat correspondence that can best be understood by examining Fig. 1.4. Electrodes are placed in the colliculus through which first the location of the receptive field of neurons is...