![]()
Chapter 1
Introduction to SOFCs
Subhash C. Singhal and Kevin Kendall
1.1 Background
Solid oxide fuel cells (SOFCs) are the most efficient devices yet invented for conversion of chemical fuels directly into electrical power. Originally the basic ideas and materials were proposed by Nernst and his colleagues [1–3] in Gottingen at the end of the nineteenth century, as described in Chapter 2, but considerable advances in theory and experiment are still being made over 100 years later.
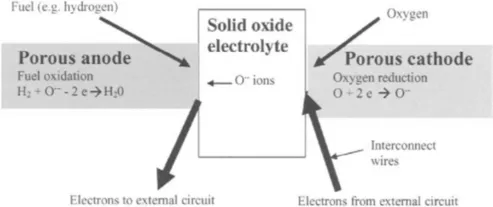
Figure 1.1 shows an SOFC scheme. It contains a solid oxide electrolyte made from a ceramic such as yttria-stabilised zirconia (YSZ) which acts as a conductor of oxide ions at temperatures from 600 to 1000°C. This ceramic material allows oxygen atoms to be reduced on its porous cathode surface by electrons, thus being converted into oxide ions, which are then transported through the ceramic body to a fuel-rich porous anode zone where the oxide ions can react, say with hydrogen, giving up electrons to an external circuit as shown in Figure 1.1. Only five components are needed to put such a cell together: electrolyte, anode, cathode and two interconnect wires.
Figure 1.1 Schematic of solid oxide fuel cell (SOFC)
This is almost magical in its elegance and simplicity, and it is astonishing that this process has not yet been commercialised to supplant the inefficient and polluting combustion heat engines which currently dominate our civilization. Largely, this failure has stemmed from a lack of materials knowledge and the absence of chemical engineering skills necessary to develop electrochemical technology. Our belief is that this knowledge and expertise is now emerging rapidly. The purpose of this book is to present this up-to-date knowledge in order to facilitate the inventions, designs and developments necessary for commercial applications of solid oxide fuel cells.
An essential aspect of SOFC design and application is the heat produced by the electrochemical reaction, not shown in Fig. 1.1. As Chapter 3 shows, heat is inevitably generated in the SOFC by ohmic losses, electrode overpotentials etc. These losses are present in all designs and cannot be eliminated but must be integrated into a heat management system. Indeed, the heat is necessary to maintain the operating temperature of the cells. The benefit of the SOFC over competing fuel cells is the higher temperature of the exhaust heat which makes its control and utilization simple and economic.
Because both electricity and heat are desirable and useful products of SOFC operation, the best applications are those which use both, for example residential combined heat and power, auxiliary power supplies on vehicles, and stationary power generation from coal which needs heat for gasification. A residential SOFC system can use this heat to produce hot water, as currently achieved with simple heat exchangers. In a vehicle the heat can be used to keep the driver warm. A stationary power system can use the hot gas output from the SOFC to gasify coal, or to drive a heat engine such as a Stirling engine or a gas turbine motor.
These ideas, from fundamentals of SOFCs through to applications, are expanded in the sections below to outline this book’s contents.
1.2 Historical Summary
The development of the ideas mentioned above has taken place over more than a century. In 1890, it was not yet clear what electrical conduction was. The electron had not quite been defined. Metals were known to conduct electricity in accord with Ohm’s law, and aqueous ionic solutions were known to conduct larger entities called ions. Nernst then made the breakthrough of observing various types of conduction in stabilised zirconia, that is zirconium oxide doped with several mole per cent of calcia, magnesia, yttria, etc. Nernst found that stabilised zirconia was an insulator at room temperature, conducted ions in red hot conditions, from 600 to 1000°C and then became an electronic and ionic conductor at white heat, around 1500°C. He patented an incandescent electric light made from a zirconia filament and sold this invention which he had been using to illuminate his home [1–3]. He praised the simultaneous invention of the telephone because it enabled him to call his wife to switch on the light device while he travelled back from the university. The heat-up time was a problem even then [4].
The zirconia lighting filament was not successful in competing with tungsten lamps and Nernst’s invention languished until the late 1930s when a fuel cell concept based on zirconium oxide was demonstrated at the laboratory scale by Baur and Preis [5]. They used a tubular crucible made from zirconia stabilised with 15 wt% yttria as the electrolyte. Iron or carbon was used as the anode and magnetite (Fe304) as the cathode. Hydrogen or carbon monoxide was the fuel on the inside of the tube and air was the oxidant on the outside. Eight cells were connected in series to make the first SOFC stack. They obtained power from the device and speculated that this solid oxide fuel cell could compete with batteries. But several improvements were necessary before this would be possible. For example, the electrolyte manufacturing process was too crude and needed optimising, especially to make the electrolyte thinner to reduce the cell resistance from around 2Ω. In addition, the electrodes were inadequate, especially the cathode Fe304 which readily oxidised. Also, the power density was small with the stacking arrangement used, the connections between many cells had to be developed, and the understanding of fuel reactions and system operation needed much attention.
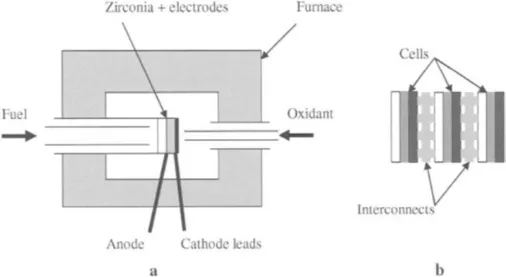
It was not until the 1950s that experiments began on pressed or tape-cast discs of stabilised zirconia when a straightforward design of test system was developed which is still in use today. The essentials of the apparatus are shown in Figure 1.2a. A flat disc of stabilised zirconia, with anode and cathode on its two sides, was sealed to a ceramic tube and inserted in a furnace held at red heat [6]. A smaller diameter tube was inserted into the ceramic tube to bring fuel to the anode, and another tube brought oxidant gas to the cathode side. Current collector wires and voltage measurement probes were brought out from the electrode surfaces. Once a flat plate of electrolyte had been used, it was easy to see how the flat plate voltaic stack could be built up with interconnecting separator plates to build a realistic electrochemical reactor, as shown in Figure 1.2b. The interconnect plate is essentially made from the anode current collector and the cathode current collector joined together into one sheet, thus combining the two components. Additionally, the interconnector can contain gas channels which supply fuel to the anode and oxidant to the cathode as well as electrically connecting the anode of one cell to the cathode of the next.
It turned out that there were several problems with flat plate stacks as they were made larger to generate increased power, including sealing around the edges and thermal expansion mismatch which caused cracking. Consequently, tubular designs have had greater success in recent years. However, the configuration of Figure 1.2 has been prominent in zirconia sensors, discussed in the next section, which are now manufactured in large numbers.
1.3 Zirconia Sensors for Oxygen Measurement
An SOFC in reality already exists on every automobile: it is the oxygen sensor device which sits in the exhaust manifold in order to control the oxygen content of the effluent mixture entering the exhaust catalyst. The composition of the effluent mixture must be controlled to near stoichiometric if the catalyst is to operate at its optimum performance. Yttria-stabilised zirconia (YSZ) is generally used as the electrolyte because this uniquely detects oxygen, and platinum is normally painted on its surface using proprietary inks to provide the electrodes.
In the original design, which was used from the 1970s, the configuration was similar to that of Baur and Preis [5]. A thimble of YSZ, containing typically 8wt% yttria, was pressed from powder and fired to 1500°C to densify it. Platinum electrodes were applied and the unit then fixed in a steel plug which could be screwed into the car exhaust manifold, so that the YSZ +anode was protruding into the hot gases. Air was used as the oxygen reference on the cathode side. A wire connection supplied the voltage from the inner electrode to the engine management system, while the other electrode was grounded to the chassis.
Once the exhaust warmed up, above 600°C, the voltage from the sensor reflected the oxygen concentration in the exhaust gas stream. This voltage varied with the logarithm of oxygen level, giving the characteristic λ-shap...