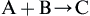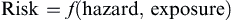Underpinnings of Green Chemistry
With the benefit of hindsight, it may be easy to say that green chemistry and engineering began with the simple idea that chemistry, as it is generally practiced, needs to change. Green chemistry and engineering evolved from the ideas and practices of pollution prevention and waste minimization that were established in the 1980s. It also had additional help because industry had to respond to an array of legislation that governments promulgated to reduce and eliminate the generation of toxics and their emissions to the air, water, and on to the land [1]. Green chemistry and engineering, if it is practiced correctly, is the ultimate form of resource consumption minimization and pollution source reduction and control. It should not be a surprise that if one is reducing resource consumption and minimizing pollution, there will be great economic benefits that result.
From a chemist's perspective, one generally writes a simple equation (Eq. [1.1]) to depict a chemical reaction:
The problem in writing a reaction this way is that only a small part of the overall chemical story is represented. Very rarely can a chemist take two chemicals, mix them in their pure states, and expect them to react quantitatively to form a single product. Chemistry, like most of life, is messy and rarely this simple. To start with, chemicals have to be contained in some way; they need some kind and type of reaction space that keeps the reactants close to one another so they can react and at the desired reaction temperature and pressure. Chemists do not often think about the types and places where these reactions will proceed and how to optimize thermal conditions (heating and cooling) and mass transfer phenomena (e.g., mixing, flow, etc.); that is the province of the chemical engineer. But chemists do know that for most reactions to proceed, reactants need to be dissolved in a solvent with unique properties that are beneficial to the reaction and along with other reagents (e.g., acids, bases, salts, phase transfer agents, etc.), catalysts, or the need for other chemicals to be added to get A and B to react in a timely fashion. Then there is a need to either isolate the product C or contain it as an intermediate that is to be used in the next step of a chemical synthesis or manufacturing process. Isolation of the desired product C is not a trivial step and many times will require the use of a filter aid, temperature swing, or an antisolvent to shift the chemical equilibrium and force the formation of a product-containing precipitate. Sometimes, as a result of these actions, an emulsion is formed that requires the chemist to add another chemical, like a surfactant, or another solvent or salt to break the emulsion. This results in another step or series of steps to the already growing process sequence.
While the resulting end product is obtained, all these additional chemicals, catalysts, solvents, regents, etc. generally end up as waste, with each needing additional process step(s) to separate, purify, recycle, remediate, or dispose of in some appropriate fashion. These additional actions require and add to the overall energy use and environmental footprint of that once simple chemical reaction, as identified in Eq. [1.1]. It is for these reasons that people started thinking more about how pollution could be minimized and prevented through changes in chemistry and chemical processes. What this typically meant prior to the 1990s was that in practice no one changed the chemistry occurring within the chemical processes; they just attempted to treat the waste to make it less impactful before discharging into the environment as a gas, vapor, liquid, or solid. As a strategy, it is certainly possible to continue managing and treating wastes of all kinds, but this approach bears significant costs, and even if something is less harmful, it may still have a variety of potential impacts to humans and the environment. It is also true that there are valuable resources within these waste streams that are being irreversibly lost to use by these management and treatment methods.
How Did We Get Here?
If one thinks about the varieties of chemicals used in many chemical processes, it is likely that a majority of them have the propensity to cause some type of toxic effect to humans [2] and to a variety of organisms if they are released into the surrounding environment. The inherent toxicity associated with chemicals has the potential to result in considerable economic costs, as well as environmental and human health impacts as chemicals are manufactured, transported, and stored prior to their use in a chemical process [3]. Mixing these process chemicals together, i.e., chemicals that are not reactants, in a manufacturing process usually does not reduce their toxicity. In many cases these process chemicals retain their toxic effects and this may lead to potentially multiple toxic effects to be managed within a waste stream.
It could be argued that most chemists generally take it for granted that the chemicals and materials they work with are hazardous and very reactive. They learn to control this reactivity in a variety of ways, usually through controlling conditions in a laboratory and by using small quantities of each chemical, activities that are feasible at small scale for minimal economic cost. It is that tendency for chemicals to react, combined with other specific and unique properties, that can lead to many potentially harmful effects or impacts that create problems for human and environmental organisms. Chemists rely on the fact that chemicals react with each other, and they purposefully choose chemicals that react quickly, robustly, predictably, and quantitatively [4–6]. But, for the nonchemists in the world, the idea of working with materials that can cause explosions, fires, or which in most cases have a variety of toxicity concerns is not something most people aspire to do. Nor are many consumers very comfortable with the idea of these chemicals ending up in a commercial product they use in their home or wear. Chemists may be amused by the naivety most of society has about chemicals, but the fact is that many consumers have an unhealthy fear of chemicals [7], despite the fact that they are heavily dependent on chemicals and the products made from chemicals.
This fear of chemicals in recent years has spurred considerable talk among government regulators and industry about the design and use of safer chemical alternatives [8]. On the surface of it, this sounds like something everyone can get behind. In practice it is not so easy to implement, and basing chemical use on the inherent hazard properties of a chemical compound or a mixture of chemicals creates a major problem for chemistry, as it is practiced today. Following this line of thinking to its logical extreme, a company could not make or sell a great number of commercial products that are critical to maintaining the lifestyles of those people living in the developed and developing world, and critical to the continuance of modern society. Nor could this approach allow a company to maintain their market position within their sector and be economically sustainable. Go one or two steps upstream in the value chain of any product and you will encounter a reasonable number of hazardous chemicals or by-products that are present as a consequence of the chemical reactions used in making the product.
Hazard and Risk
There are at least two strategies to help chemists address this particular problem. The first and traditional way industry approaches this problem is to merely say that industry needs to do a better job of communicating about the benefits of chemicals or products made from chemicals. A portion of communicating about these benefits is for industry to better help people understand the concept of chemical risk assessment [9–12]. This will aid in understanding that there is not a strict reliance upon basing acceptable chemical use and selection solely upon considering the inherent hazards of any given chemical. Risk is generally understood as follows:
where exposure is determined by the physical properties of the compound and the frequency and duration of the exposure to that compound. In the case of physical hazards like explosivity, exposure is further characterized by severity and probability. Developing a better understanding of risk allows one to accept that a person can work with hazardous chemicals, and exposures can be controlled to reduce or eliminate the impacts of hazardous chemicals in products.
The second strategy is to look at hazard as an opportunity. This tactic may sound a bit strange at first, but all that manufacturers want to do is produce a product or a chemical that performs its desired function and make a profit doing just that. For example, consumers want a material or product to have a particular color, or perhaps it is a protective coating, or a car that has good gas mileage, so a new structural composite that reduces the vehicle weight while providing greater crash protection would be desirable. If one thinks about color for a moment, color is imparted to different products sometimes as a dye (e.g., textiles), in other applications as a pigment (e.g., printing or coatings), or in other applications as a physical struct...