
- 344 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Green Chemistry and Engineering
About this book
Chemical processes provide a diverse array of valuable products and materials used in applications ranging from health care to transportation and food processing. Yet these same chemical processes that provide products and materials essential to modern economies, also generate substantial quantities of wastes and emissions. Green Chemistry is the utilization of a set of principles that reduces or eliminate the use or generation of hazardous substances in design. Due to extravagant costs needed to managing these wastes, tens of billions of dollars a year, there is a need to propose a way to create less waste. Emission and treatment standards continue to become more stringent, which causes these costs to continue to escalate.
Green Chemistry and Engineering describes both the science (theory) and engineering (application) principles of Green Chemistry that lead to the generation of less waste. It explores the use of milder manufacturing conditions resulting from the use of smarter organic synthetic techniques and the maintenance of atom efficiency that can temper the effects of chemical processes. By implementing these techniques means less waste, which will save industry millions of dollars over time.
- Chemical processes that provide products and materials essential to modern economies generate substantial quantities of wastes and emissions, this new book describes both the science (theory) and engineering (application) principles of Green Chemistry that lead to the generation of less waste
- This book contains expert advise from scientists around the world, encompassing developments in the field since 2000
- Aids manufacturers, scientists, managers, and engineers on how to implement ongoing changes in a vast developing field that is important to the environment and our lives
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER 1
Introduction
Publisher Summary
Green chemistry has become the important philosophy since the 21−st century. Increased global competition has forced industries to look at green routes for achieving efficient manufacturing processes. As a result, chemical and allied industries have taken it seriously due to societal and governmental pressures with respect to environmental issues. Measuring the greenness of a process is a very difficult task. Several indicators are now being defined to measure the process characteristics. There are several levels in the green chemistry hierarchy, and each level is interested in certain indicators. Besides considering an ideal process and product, one needs to include an ideal user as well. The user can support the green chemistry initiative by being responsible in product selection, usage, and disposal. A product recycle could lead to five times more employment than a remediation operation. Of the four Rs—Reduce, Recycle, Reuse, and Remediate—the first two are part of the green chemistry principles. The third R describes a responsible user; and the fourth R should be the last option. This chapter outlines the latest developments in green chemistry and green process technologies, with relevant industrial examples.
The chemical industry accounts for 7% of global income and 9% of global trade, adding up to US$1.5 trillion in sales in 1998, with 80% of the world’s output produced by 16 countries. Production is projected to increase 85% by 2020 compared to the 1995 levels. This will be in pace with GDP growth in the United States, but at twice the per capita intensity. There will be strong market penetration by countries other than these 16, especially in commodity chemicals (OECD, 2001). Over the past half-century, the largest growth in volume of any category of materials has been in petrochemical-based plastics; and in terms of revenue it was pharmaceuticals. The latter, in the past two decades, has become number one. Overall production has shifted from predominantly commodity chemicals to fine and specialty chemicals, and now it is the life sciences. In the United States, the chemical industry contributes 5% of GDP and adds 12% of the value to GDP by all U.S. manufacturing industries, and it is also the nation’s top exporter (Lenz and Lafrance, 1996). This information speaks volumes about the importance of chemical industries in our day-to-day life and in supporting the nation’s economy. But it is plagued with several problems, such as running out of petrochemical feedstock, environmental issues, toxic discharge, depletion of nonrenewable resources, short-term and long-term health problems due to exposure of the public to chemicals and solvents, and safety concerns, among others.
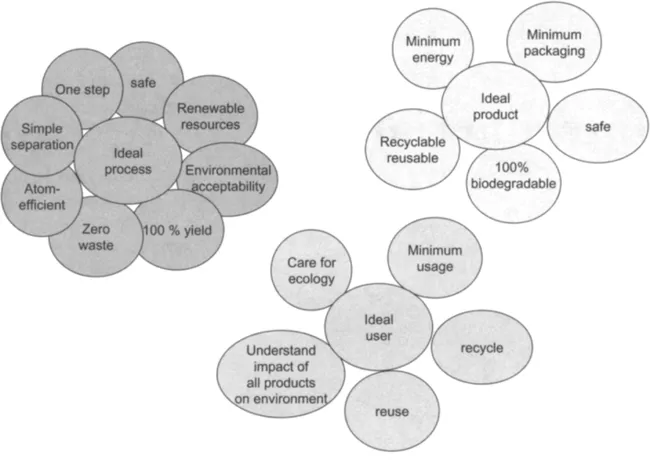
About 7.1 billion pounds of more than 650 toxic chemicals were released to the environment in 2000 by the United States alone (Environmental Protection Agency, 2002, www.epa.gov). This inventory represents only a small fraction of the approximately 75,000 chemicals in commercial use in the United States. The health and environmental effects of many chemicals are not known completely, even though some have been in use for several decades. The U.S. industry spends about $10 billion per year on environmental R&D. An ideal manufacturing process and an ideal product should have certain criteria, which are depicted in Fig. 1.1. An ideal process is simple, requires one step, is safe, uses renewable resources, is environmentally acceptable, has total yield, produces zero waste, is atom-efficient, and consists of simple separation steps. An ideal product requires minimum energy and minimum packaging, is safe and 100% biodegradable, and is recyclable. Generally, the public focuses on the process and product, paying very little attention to the “ideal user.” Figure 1.1 also lists an ideal user. An ideal user cares for the environment, uses minimal amounts, recycles, reuses, and understands a product’s environmental impact. In addition, an ideal user encourages “green” initiatives.
Definition of Green Chemistry
Green chemistry involves a reduction in, or elimination of, the use of hazardous substances in a chemical process or the generation of hazardous or toxic intermediates or products. This includes feedstock, reagents, solvents, products, and byproducts. It also includes the use of sustainable raw material and energy sources for this manufacturing process (Anastas and Warner, 1998; Anastas and Lankey, 2000, 2002; Anastas et al., 2001). A responsible user is also required to achieve the goals of green chemistry. The U.S. Presidential Green Chemistry Challenge, March 1995, defines green chemistry as,
the use of chemistry for source reduction or pollution prevention, the highest tier of the risk management hierarchy as described in the Pollution Prevention Act of 1990. More specifically, green chemistry is the design of chemical products and processes that are more environmentally benign.
Green and sustainable chemistry, a new concept that arose in the early 1990s, gained wider interest and support only at the turn of the millennium. Green and sustainable chemistry concerns the development of processes and technologies that result in more efficient chemical reactions that generate little waste and fewer environmental emissions than “traditional” chemical reactions do. Green chemistry encompasses all aspects and types of chemical processes that reduce negative impacts to human health and the environment relative to the current state-of-the-art practices (Graedel, 2001). By reducing or eliminating the use or generation of hazardous substances associated with a particular synthesis or process, chemists can greatly reduce risks to both human health and the environment.
Twelve Principles of Green Chemistry
The 12 principles of green chemistry are listed below (Clark and Macquarrie, 2002). Of course, over the years additional principles have been added to these original 12, but those could be derived from these 12 principles.
1. Prevention. It is better to prevent waste than to treat or clean it up after it has been generated in a process. This is based on the concept of “stop the pollutant at the source.”
2. Atom economy. Synthetic steps or reactions should be designed to maximize the incorporation of all raw materials used in the process into the final product, instead of generating unwanted side or wasteful products (Trost, 1991, 1995).
3. Less hazardous chemical use. Synthetic methods should be designed to use and generate substances that possess little or no toxicity to the environment and public at large.
4. Design for safer chemicals. Chemical products should be designed so that t...
Table of contents
- Cover image
- Title page
- Table of Contents
- Preface
- About the Authors …
- Chapter 1: Introduction
- Chapter 2: Newer Synthetic Methods
- Chapter 3: Catalysis and Green Chemistry
- Chapter 4: Biocatalysis: Green Chemistry
- Chapter 5: Alternate Solvents
- Chapter 6: Process and Operations
- Chapter 7: Alternate Energy Sources
- Chapter 8: Inherent Safety
- Chapter 9: Industrial Examples
- Chapter 10: Conclusions and Future Trends
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Green Chemistry and Engineering by Mukesh Doble,Ken Rollins,Anil Kumar in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.