Introduction
It has been roughly estimated that approximately 70% of all major clinical decisions involve consideration of laboratory results. In addition, approximately 40–94% of all objective health record data are laboratory results [1–3]. Undoubtedly, accurate test results are essential for major clinical decisions involving disease identification, classification, treatment, and monitoring. Factors that constitute an accurate laboratory result involve more than analytical accuracy and can be summarized as follows:
1. The right sample was collected on the right patient, at the correct time, with appropriate patient preparation.
2. The right technique was used collecting the sample to avoid contamination with intravenous fluids, tissue damage, prolonged venous stasis, or hemolysis.
3. The sample was properly transported to the laboratory, stored at the right temperature, processed for analysis, and analyzed in a manner that avoids artifactual changes in the measured analyte levels.
4. The analytical assay measured the concentration of the analyte corresponding to its “true” level (compared to a “gold standard” measurement) within a clinically acceptable margin of error (the total acceptable analytical error (TAAE)).
5. The report reaching the clinician contained the right result, together with interpretative information, such as a reference range and other comments, aiding clinicians in the decision-making process.
Failure at any of these steps can result in an erroneous or misleading laboratory result, sometimes with adverse outcomes. For example, interferences with point-of-care glucose testing due to treatment with maltose-containing fluids have led to failure to recognize significant hypoglycemia and to mortality or severe morbidity [4].
Errors in the Clinical Laboratory
Errors can occur in all the steps in the laboratory testing process, and such errors can be classified as follows:
1. Pre-analytical steps, encompassing the decision to test, transmission of the order to the laboratory for analysis, patient preparation and identification, sample collection, and specimen processing.
2. Analytical assay, which produces a laboratory result.
3. Post-analytical steps, involving the transmission of the laboratory data to the clinical provider, who uses the information for decision making.
Although minimization of analytical errors has been the main focus of developments in laboratory medicine, the other steps are more frequent sources of erroneous results. An analysis indicated that in the laboratory, pre-analytical errors accounted for 62% of all errors, with post-analytical representing 23% and analytical 15% of all laboratory errors [5]. The most common pre-analytical errors included incorrect order transmission (at a frequency of approximately 3% of all orders) and hemolysis (approximately 0.3% of all samples) [6]. Other frequent causes of pre-analytical errors include the following:
• Patient identification error
• Tube-filling error, empty tubes, missing tubes, or wrong sample container
• Sample contamination or collected from infusion route
• Inadequate sample temperature.
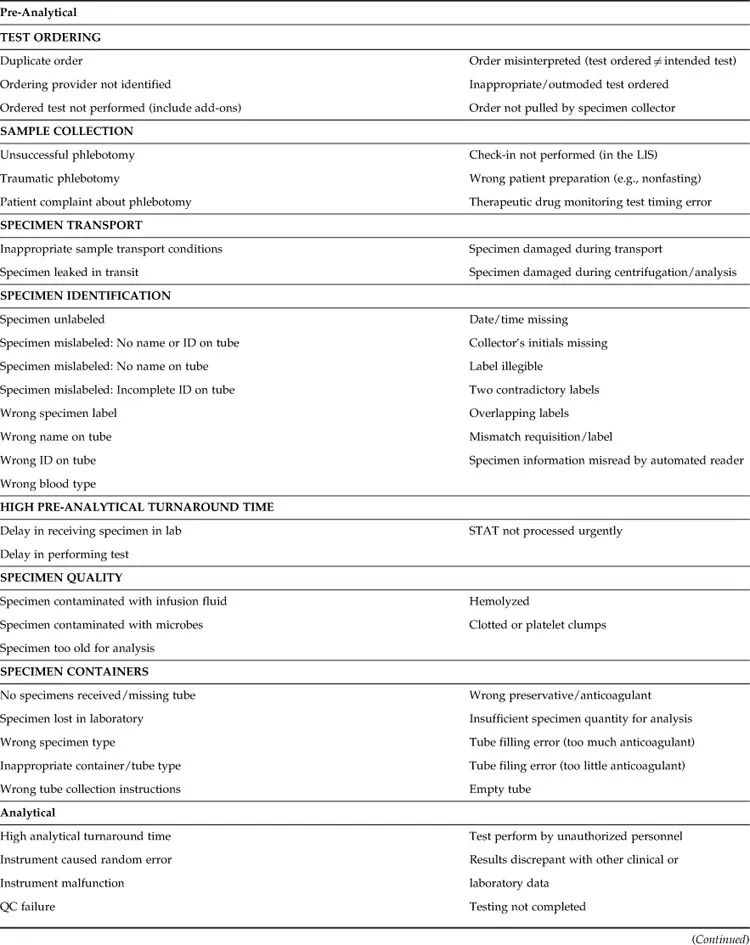
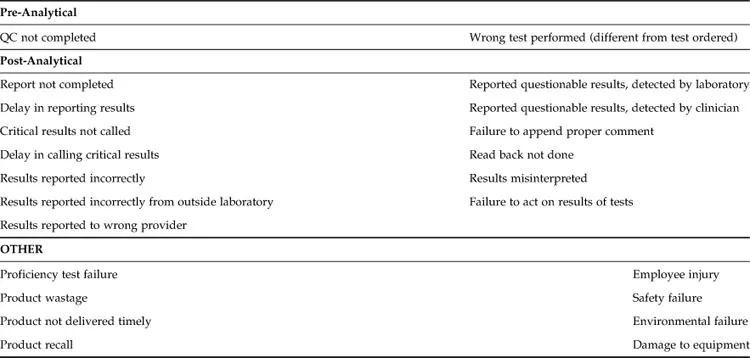
Table 1.1 provides a complete list of errors, including pre-analytical, analytical, and post-analytical errors, that may occur in clinical laboratories. Particular attention should be paid to patient identification because errors in this critical step can have severe consequences, including fatal outcomes, for example, due to transfusion reactions. To minimize identification errors, health care systems are using point-of-care identification systems, which typically involve the following:
1. Handheld devices connected to the laboratory information systems (LIS) that can objectively identify the patient by scanning a patient-attached bar code, typically a wrist band.
2. Current laboratory orders can be retrieved from the LIS.
3. Ideally, collection information, such as correct tube types, is displayed in the device.
4. Bar-coded labels are printed at the patient’s side, minimizing the possibility of misplacing the labels on the wrong patient samples.
Table 1.1
Types of Error in the Clinical Laboratory
Analytical errors are mostly due to interference or other unrecognized causes of inaccuracy, whereas instrument random errors accounted for only 2% of all laboratory errors in one study [5]. According to that study, most common post-analytical errors were due to communication breakdown between the laboratory and the clinicians, whereas only 1% were due to miscommunication within the laboratory, and 1% of the results had excessive turnaround time for reporting [5]. Post-analytical errors due to incorrect transcription of laboratory data have been greatly reduced because of the availability of automated analyzers and bidirectional interfaces with the LIS [5]. However, transcription errors and calculation errors remain a major area of concern in those testing areas without automated interfaces between the instrument and the LIS. Further developments to reduce reporting errors and minimize the testing turnaround time include autovalidation of test results falling within pre-established rule-based parameters and systems for automatic paging of critical results to providers.
When classifying sources of error, it is important to distinguish between cognitive errors, or mistakes, which are due to poor knowledge or judgment, and noncognitive errors, commonly known as slips and lapses, due to interruptions in a process that is routine or relatively automatic. Whereas the first type can be prevented by increased training, competency evaluation, and process aids such as checklists or “cheat sheets” summarizing important steps in a procedure, noncognitive errors are best addressed by process improvement and environment re-engineering to minimize distractions and fatigue. Furthermore, it is useful to classify adverse occurrences as active—that is, the immediate result of an action by the person performing a task—or as latent or system errors, which are system deficiencies due to poor design or implementation that enable or amplify active errors. In one study, only approximately 11% of the errors were cognitive, all in the pre-analytical phase, and approximately 33% of the errors were latent [5]. Therefore, the vast majority of errors are noncognitive slips and lapses performed by the personnel directly involved in the process. Importantly, 92% of the pre-analytical, 88% of analytical, and 14% of post-analytical errors were preventable. Undoubtedly, human factors, engineering, and ergonomics—optimization of systems and process redesigning to include increased automation and user-friendly, simple, and rule-based functions, alerts, barriers, and visual feedback—are more effective than education and personnel-specific solutions to consistently increase laboratory quality and minimize errors.
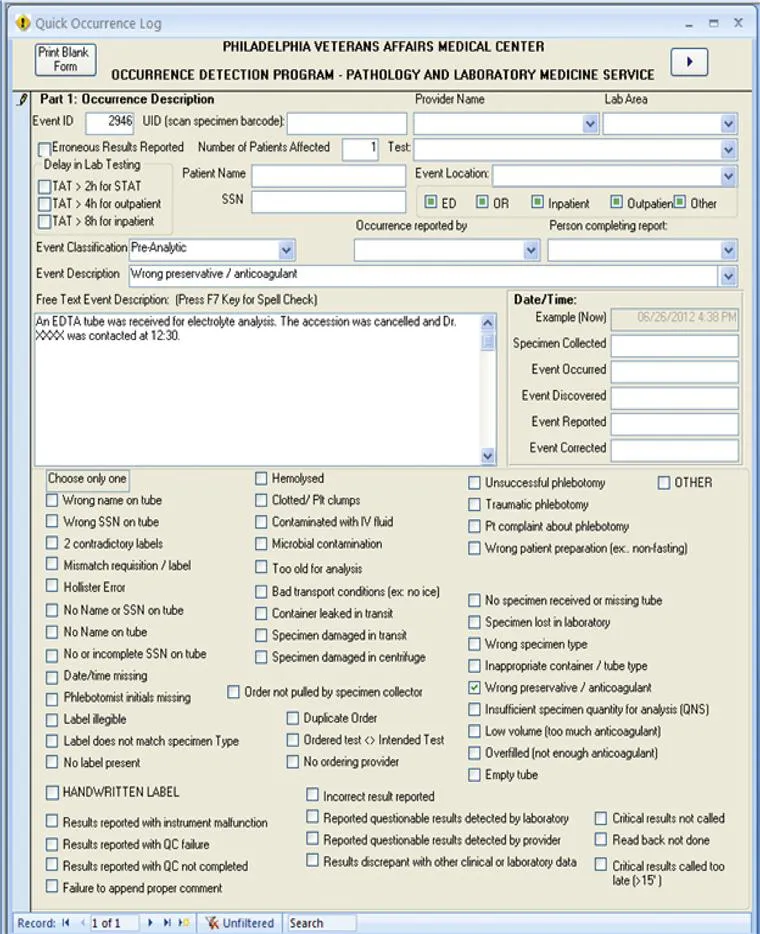
Immediate reporting of errors to a database accessible to all the personnel in the health care system, followed by automatic alerts to quality management personnel, is important for accurate tracking and timely correction of latent errors. In our experience, reporting is improved by using an online form that includes checkboxes for the most common types of errors together with free-text for additional information (Figure 1.1). Reviewers can subsequently classify errors as cognitive/noncognitive, latent/active, and internal to laboratory/internal to institution/external to institution; determine and classify root causes as involving human factors (e.g., communication and training or judgment), software, or physical factors (environment, instrument, hardware, etc.); and perform outcome analysis. Outcomes of errors can be classified as follows:
1. Target of error (patient, staff, visitors, or equipment).
2. Actual outcome on a severity scale (from unnoticed to fatal) and worst outcome likelihood if error was not intercepted, because many errors are corrected before they cause injury. Errors with significant outcomes or likelihoods of adverse outcomes should be discussed by quality management staff to determine appropriate corrective actions and process improvement initiatives.
Figure 1.1 Example of an error reporting form for the clinical laboratory.
Clearly, efforts to improve accuracy of laboratory results should encompass all of the steps of the testing cycle, a concept expressed as “total testing process” or “brain-to-brain testing loop” [7]. Approaches to achieve error minimization derived from industrial processes include total quality management (TQM); [8] lean dynamics and Toyota production systems; [9] root cause analysis (RCA); [10] health care failure modes and effects analysis (HFMEA); [11,12] failure review analysis and corrective action system (FRACAS) [13]; and Six Sigma [14,15], which aims at minimizing the variability of products such that the statistical frequency of errors is below 3.4 per million. A detailed description of these approaches is beyond the scope of this book, but laboratorians and quality management specialists should be familiar with these principles for efficient, high-quality laboratory operation [8].