- 528 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
NMR Spectroscopy in Pharmaceutical Analysis
About this book
For almost a decade, quantitative NMR spectroscopy (qNMR) has been established as valuable tool in drug analysis. In all disciplines, i. e. drug identification, impurity profiling and assay, qNMR can be utilized. Separation techniques such as high performance liquid chromatography, gas chromatography, super fluid chromatography and capillary electrophoresis techniques, govern the purity evaluation of drugs. However, these techniques are not always able to solve the analytical problems often resulting in insufficient methods. Nevertheless such methods find their way into international pharmacopoeias. Thus, the aim of the book is to describe the possibilities of qNMR in pharmaceutical analysis. Beside the introduction to the physical fundamentals and techniques the principles of the application in drug analysis are described: quality evaluation of drugs, polymer characterization, natural products and corresponding reference compounds, metabolism, and solid phase NMR spectroscopy for the characterization drug substances, e.g. the water content, polymorphism, and drug formulations, e.g. tablets, powders. This part is accompanied by more special chapters dealing with representative examples. They give more detailed information by means of concrete examples.- Combines theory, techniques, and concrete applications—all of which closely resemble the laboratory experience- Considers international pharmacopoeias, addressing the concern for licensing- Features the work of academics and researchers, appealing to a broad readership
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part I. Fundamentals and Techniques
Chapter 1. Principles in NMR Spectroscopy
1. Short History
2. The NMR Experiment
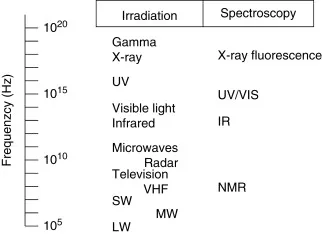
Figure 1. Energy levels of different spectroscopic methods.
2.1. Excitation, relaxation and sensitivity
Table of contents
- Brief Table of Contents
- Table of Contents
- Copyright Page
- Preface
- List of Contributors
- List of Editors
- Part I. Fundamentals and Techniques
- Part II. General Applications
- Part III. Special Applications
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app