
- 600 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Hydrogen fuel cells are emerging as a major alternative energy source in transportation and other applications. Central to the development of the hydrogen economy is safe, efficient and viable storage of hydrogen. Solid-state hydrogen storage: Materials and chemistry reviews the latest developments in solid-state hydrogen storage.Part one discusses hydrogen storage technologies, hydrogen futures, hydrogen containment materials and solid-state hydrogen storage system design. Part two reviews the analysis of hydrogen interactions including structural characterisation of hydride materials, neutron scattering techniques, reliably measuring hydrogen uptake in storage materials and modelling of carbon-based materials for hydrogen storage. Part three analyses physically-bound hydrogen storage with chapters on zeolites, carbon nanostructures and metal-organic framework materials. Part four examines chemically-bound hydrogen storage including intermetallics, magnesium hydride, alanates, borohydrides, imides and amides, multicomponent hydrogen storage systems, organic liquid carriers, indirect hydrogen storage in metal ammines and technological challenges in hydrogen storage.With its distinguished editor and international team of contributors, Solid-state hydrogen storage: Materials and chemistry is a standard reference for researchers and professionals in the field of renewable energy, hydrogen fuel cells and hydrogen storage.
- Assesses hydrogen fuel cells as a major alternative energy source
- Discusses hydrogen storage technologies and solid-state hydrogen storage system design
- Explores the analysis of hydrogen interactions including reliably measuring hydrogen uptake in storage materials
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part I
Introduction
1
Hydrogen storage technologies
G. WALKER, University of Nottingham, UK
Publisher Summary
This chapter presents an introduction to the potential applications for hydrogen storage and provides an overview of the different technologies available for storing hydrogen. The various applications have different requirements in terms of the mass of hydrogen needed, volumetric capacity, gravimetric capacity and cost. The conventional hydrogen storage technologies are compressed gas and liquid storage. These hydrogen storage technologies are the current state-of-the-art, but more compact means of storing hydrogen are needed for portable and mobile applications, solid-state hydrogen storage materials would appear to be the most promising solution. The chapter describes these technologies and discusses alternative hydrogen storage material technologies. These materials can be split into three categories: physically bound hydrogen, where the hydrogen gas is physisorbed to a high surface area substrate; chemically bound hydrogen, where the hydrogen has formed a chemical compound with the substrate, for example, metal hydrides and complex hydrides, and the hydrogen is released through a thermal decomposition; and lastly hydrolytic evolution of hydrogen (sometimes referred to as chemical hydrides), a variation on chemically bound hydrogen where the hydrogen is released through a chemical reaction, typically a hydrolysis reaction, for example, the hydrolysis of metal powders and hydrides.
1.1 Introduction
There are significant concerns about the rising level of CO2 emissions and the impact this is having on our environment (IPCC, 2007). The main source of the increase in CO2 is from our increasing energy demands. Global energy needs are expanding rapidly: in 1973 the global demand for energy was 6128 Mtoe (million tonnes of oil equivalent), which almost doubled over the following three decades to 11435 Mtoe in 2005 (IEA, 2007a). Of this, the percentage contribution from countries in the Organization for Economic Cooperation and Development (OECD) fell from 61.3% to 48.5% while that for Asia and the Middle East more than doubled from 13.8% to 30.8% (IEA, 2007a). If energy policies do not change, energy scenarios predict demand will reach 17 100 Mtoe by 2030, with the developing countries contributing to 74% of that increase (IEA, 2007b). Fossil fuels currently account for 81% of the world’s energy needs and the scenario for 2030 shows little change in this percentage because of the reliance of the developing economies on coal. If we consider the energy consumption per capita we see a different picture emerging. Asia and the Middle East energy consumption is 1.0 toe/capita and that for OECD countries is 4.7 toe/capita. These results help illustrate that the ‘problem’ is not simply the increasing demand from the developing countries, but that the demand per capita for developed nations is almost five times that of the developing nations. This is a global problem, with developed countries needing to reduce their energy consumption per capita while enabling developing economies to expand (and the inevitable increase in energy consumption that that will result in).
In addition to limiting energy consumption, low-carbon energy technologies are needed to further reduce CO2 emissions and alternatives to burning fossil fuels are required. Hydrogen has a high calorimetric value, with a lower heating value (LHV, i.e. the heat released upon combustion without recovery of the latent heat of vaporisation of any water produced) of 120 MJ kg−1, compared with petrol, which is approximately a third of this at 43 MJ kg−1. Hydrogen can be used for power generation either by combustion in for example an internal combustion engine (ICE), producing mechanical power, or electrochemically by using a fuel cell, producing electrical power. In both examples the hydrogen reacts with oxygen to form water. For fuel cells this is the only emission, but with ICE, NOx can be formed if the combustion is too hot, hence ICE engines are often run lean to avoid this. Although hydrogen is a very abundant element, it exists on Earth primarily in combination with oxygen, as water. It can, though, be generated in a number of ways, such as electrolysis, reforming, fermentation and direct splitting of water either thermochemically or photocatalytically. If a renewable source of energy is used, the hydrogen will be CO2 neutral. If fossil fuels are used then carbon capture and sequestration will be needed for there to be a significant CO2 reduction. Hydrogen is thus a versatile energy carrier and is seen as an important part of the solution to lowering CO2 emissions.
Hydrogen fuel cells have a range of potential applications as indicated in Fig. 1.1, operating from a few watts up to gigawatts. Hydrogen microfuel cells have great potential to power portable electronics such as laptops and mobile phones. The superior energy density of a hydrogen polymer electrolyte membrane (PEM) microfuel cell compared with a lithium ion rechargeable battery opens the way to longer battery lifetimes. Small fuel cells or ICE generation sets (0.1–1 kW size) offer back-up power when disconnected from the national grid (either from outages or when working in the field). For mobile applications, fuel cells could provide auxiliary power for electrical units such as air conditioning and refrigerators (e.g. for food delivery vehicles). Both PEM fuel cells and ICEs are competing technologies for the vehicle powertrain. There is also interest in using hydrogen fuel cells for distributed generation for domestic power and using solid oxide fuel cells or combined cycle gas turbines for small power stations in the MW to GW range.
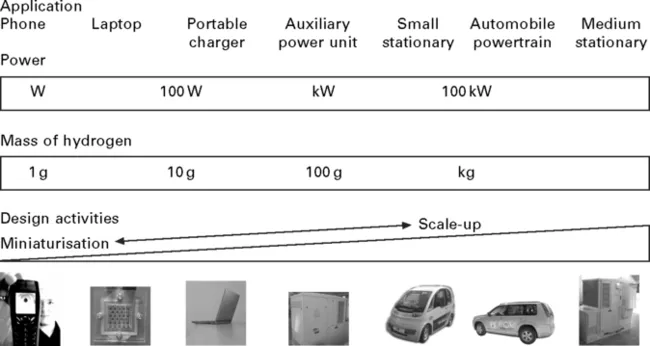
Vehicle powertrain is seen as one of the biggest potential markets for hydrogen, replacing the use of petrol and diesel, but automotive hydrogen storage is also one of the most challenging applications. Transport accounts for 30.3% of global energy use (IEA, 2007a) and is the source of a significant amount of CO2 emissions. Hydrogen, battery or most likely hybrid hydrogen/battery vehicles are the leading contenders for zero emission vehicles, an attraction for hydrogen vehicles is the combined high energy density and durability of hydrogen fuel cells in comparison with current battery technologies. The challenge with hydrogen is that it is a low-density gas and it is difficult to efficiently store enough hydrogen on-board a vehicle to give the vehicle an adequate range, e.g. c. 5 kg of hydrogen for a range of 500 km (Schlapbach and Züttel, 2001).
In the United States, the Department of Energy has set tough targets for the development of hydrogen vehicles (DOE, 2006). The targets for hydrogen storage systems (i.e. this includes the container and any balance-of-plant) are gi...
Table of contents
- Cover image
- Title page
- Table of Contents
- Related titles
- Copyright
- Contributor contact details
- Preface
- Part I: Introduction
- Part II: Analysing hydrogen interactions
- Part III: Physically bound hydrogen storage
- Part IV: Chemically bound hydrogen storage
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Solid-State Hydrogen Storage by Gavin Walker in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Renewable Power Resources. We have over one million books available in our catalogue for you to explore.