
eBook - ePub
Aluminum-Lithium Alloys
Processing, Properties, and Applications
- 608 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Aluminum-Lithium Alloys
Processing, Properties, and Applications
About this book
Because lithium is the least dense elemental metal, materials scientists and engineers have been working for decades to develop a commercially viable aluminum-lithium (Al-Li) alloy that would be even lighter and stiffer than other aluminum alloys. The first two generations of Al-Li alloys tended to suffer from several problems, including poor ductility and fracture toughness; unreliable properties, fatigue and fracture resistance; and unreliable corrosion resistance.
Now, new third generation Al-Li alloys with significantly reduced lithium content and other improvements are promising a revival for Al-Li applications in modern aircraft and aerospace vehicles. Over the last few years, these newer Al-Li alloys have attracted increasing global interest for widespread applications in the aerospace industry largely because of soaring fuel costs and the development of a new generation of civil and military aircraft. This contributed book, featuring many of the top researchers in the field, is the first up-to-date international reference for Al-Li material research, alloy development, structural design and aerospace systems engineering.
- Provides a complete treatment of the new generation of low-density AL-Li alloys, including microstructure, mechanical behavoir, processing and applications
- Covers the history of earlier generation AL-Li alloys, their basic problems, why they were never widely used, and why the new third generation Al-Li alloys could eventually replace not only traditional aluminum alloys but more expensive composite materials
- Contains two full chapters devoted to applications in the aircraft and aerospace fields, where the lighter, stronger Al-Li alloys mean better performing, more fuel-efficient aircraft
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part I
Introduction to Al–Li Alloys
Outline
Chapter 1 Historical Development and Present Status of Aluminum–Lithium Alloys
Chapter 2 Aerostructural Design and Its Application to Aluminum–Lithium Alloys
Chapter 1
Historical Development and Present Status of Aluminum–Lithium Alloys
Edgar A. Starke Jr., Department of Materials Science and Engineering, University of Virginia, Charottesville, USA
This chapter provides a brief overview and history of the development of aluminium-lithium alloys from the earlier days of the discovery of age hardening by Alfred Wilm to its current status. It examines the progress of alloy development from simple binary alloys to the complex alloys that are currently used in aerospace systems. The driving force for this development has been the advantages gained by weight reduction of aerospace systems by replacing conventional aluminium alloys with the lower density higher modulus aluminium-lithium alloys. The problems associated with the development of these alloys and the scientific solutions to solving these problems are described.
Keywords
Al–Li alloys; history; precipitation; age hardening; deformation; ductility
Contents
1.1 Introduction
1.2 Lithium Additions to Aluminum Alloys: Early Days
1.2.1 History of the Development of the First Modern Al–Li Alloys
1.2.2 Development of Alcoa’s 2020
1.2.3 The Ductility Problem of 2020
1.2.4 Development of Al–Li Alloys in the Soviet Union
1.3 Development of Modern Aluminum–Lithium Alloys
1.3.1 The Second Generation of Aluminum–Lithium Alloys
1.3.2 Manufacturing Issues
1.3.3 Understanding the Precipitate Structure in Al–Li–X Alloys
1.3.4 The Effect of Prior Deformation on Precipitation During Ageing
1.3.5 Deformation Behavior in Aged Al–Li–X Alloys
1.3.6 Predicting Strain Localization in Al–Li–X Alloys
1.3.7 Applications and Problems of the Second-Generation Al–Li Alloys
1.3.8 The Third Generation of Aluminum–Lithium Alloys
1.3.9 Background Information That Led to Improvements in Al–Li–X Alloys
1.4 Closure
Acknowledgments
References
1.1 Introduction
Although “age hardening” was discovered in 1902 by Alfred Wilm at the Center for Scientific Research in Germany (Wilm, 1911), it was not until 1919 that the phenomenon of “age hardening” was explained (Starke and Hornbogen, 2008). Merica et al. (1919) were conducting research on Wilm’s alloy duralumin at the National Bureau of Standards in the United States and concluded that the cause of age hardening was due to precipitation from a supersaturated solid solution. They also stated that the key step in the process was a decrease in solid solubility of alloying elements with decreasing temperature and speculated that quenching from the high temperature and ageing at a lower temperature would produce a very fine dispersion of small particles within the matrix. They then introduced the idea of a critical particle size associated with maximum strengthening but made no attempt to describe how these particles resulted in the strength increase. Later, Jeffries and Archer (1921) proposed that the very fine particles formed during ageing interfered with the slip process. They recognized the crystallographic nature of slip and therefore supported the ideas proposed by Merica and his colleagues. These ideas led to searches for other alloys which could age harden, notably in the United States, Germany, and Japan in the 1920s and 1930s.
1.2 Lithium Additions to Aluminum Alloys: Early Days
Around the same time that Merica and his colleagues were explaining the phenomenon of age hardening, researchers in Germany were exploring aluminum alloys containing lithium. Considering the high solubility of lithium in aluminum at high temperatures and its decreasing solubility as the temperature is lowered, it is not surprising that lithium additions were included in these investigations. Balmuth and Schmidt (1980) discussed this work in their overview of the early development of aluminum–lithium alloys that they presented at the First International Aluminum–Lithium Conference held at Stone Mountain, GA. The first commercial aluminum alloy containing lithium was the German alloy “Scleron” that had a nominal composition of Al–12Zn–3Cu–0.6Mn–0.1Li (Reuleaux, 1924). The claims of the alloy were that it had great resistance to wear, relative cheapness, high tensile strength and resistance to corrosion and oxidation, and was superior to other aluminum alloys because it could be worked into a wide variety of forms. However, research by Assmann (1926) on the strengthening effect of lithium additions to aluminum was inconclusive, and other aluminum alloys that did not contain lithium showed better properties, so Scleron production was discontinued.
The development of modern aluminum–lithium alloys can be traced to the discovery by I.M. LeBaron in 1942 that lithium could be a major strengthening element in aluminum–copper alloys. LeBaron was granted a patent on Al–Cu–Li–Mn–Cd alloys in 1945 (LeBaron, 1945). However, the introduction of 7075 by Alcoa in 1943 established the dominance of the Al–Zn–Mg–Cu system for high-strength applications, and further work on LeBaron’s alloys were discontinued at that time. Subsequent work by Hardy and Silcock in England identified the lithium-containing strengthening phases in Al–Cu–Li alloys, and their research led to an increased interest in the alloy system (Hardy, 1955–56; Hardy and Silcock, 1955–56; Silcock, 1959–60). Hardy and Silcock contributed significantly to the scientific understanding of these complex materials.
1.2.1 History of the Development of the First Modern Al–Li Alloys
An abbreviated history of the use of lithium in aluminum alloys up to 1982 is shown in Figure 1.1 (Quist et al., 1983). A number of important events not noted in Figure 1.1 include the substantive contributions of several universities, funding agencies, and research laboratories.
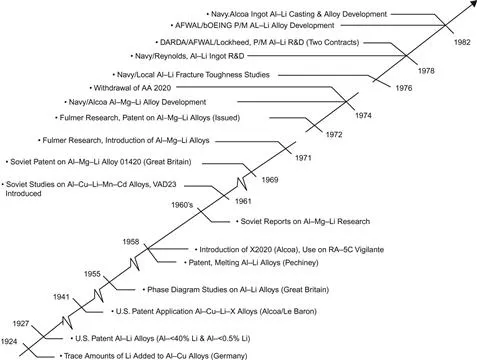
Figure 1.1 Chronological early development and use of lithium in aluminum.
1.2.2 Development of Alcoa’s 2020
In the 1950s, metallurgists at Alcoa recognized that lithium also increased the elastic modulus of aluminum and developed the high strength Al–Cu–Li alloy 2020 in 1957 (Criner, 1957, 1959). The nominal composition of 2020 was Al–4.5Cu–1.1Li–0.5Mn–0.2Cd, and in addition to high strength at 300–400°F (~150–200°C), it was claimed that the alloy was resistant to creep at these temperatures. Subsequently, 2020 was commercially produced and used on the United States Navy RA 5C Vigilante aircraft. It performed well for over 20 years without any documented failures (Balmuth and Schmidt, 1980). Aluminum–lithium alloys are attractive for aerospace applications because they have lower density and higher modulus than conventional aluminum aerospace alloys. Each weight percent of lithium lowers the density of aluminum by approximately 3% and increases the modulus by approximately 6% (Starke and Staley, 1996). However, the perceived brittleness of 2020 and the production problems thwarted further use, and it was withdrawn from commercial production in the 1960s. A plot of contemporary data reveals that, although the 2020-T6 may have exhibited low toughness, it was being used in a very strong condition and was not dissimilar in its relative performance to other high-strength aerospace alloys of that period (Figure 1.2) (Peel, 1990).
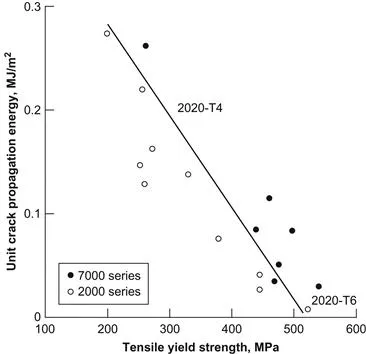
Figure 1.2 Toughness of 2020 alloy in the T4 and T6 tempers in comparison to contemporary 1960s aerospace alloys (Peel, 1990).
1.2.3 The Ductility Problem of 2020
The iron and silicon contents of a typical 2020 alloy were relatively high when compared to modern aerospace alloys. During ingot solidification and subsequent processing these impurities precipitate as the insoluble constituent phases Al12(FeMn)3Si and Al7Cu2Fe, which vary in size from 1 to 10 μm (Starke and Staley, 1996). The detrimental effect of large constituent phases on the ductility and fracture toughness of aluminum alloys was first identified in 1961 (Anderson and Quist, 1966; Quist, 1963) and documented by many investigators (Smith, 1991). Not only do these large particles initiate cracks, but they also produce an inhomogeneous strain distribution during working operations, which enhances the possibility of recrystallization during subsequent heat treatments (Cotterill and Mold, 1976). Alloy 2020 had...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Foreword
- Preface
- About the Editors
- A Note of Gratitude from the Editors
- List of Contributors
- Part I: Introduction to Al–Li Alloys
- Part II: Physical Metallurgy
- Part III: Processing Technologies
- Part IV: Mechanical Behavior
- Part V: Applications
- Appendix 1. Interconversion of Weight and Atomic Percentages of Lithium and Aluminum in Aluminum–Lithium Alloys
- Selected Conversion Factors For SI Units
- Index
- Bibliography
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Aluminum-Lithium Alloys by N Eswara Prasad,Amol Gokhale,R.J.H Wanhill in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Materials Science. We have over one million books available in our catalogue for you to explore.