
- 860 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
Handbook of Thermal Analysis and Calorimetry: Recent Advances, Techniques and Applications, Volume Six, Second Edition, presents the latest in a series that has been well received by the thermal analysis and calorimetry community. This volume covers recent advances in techniques and applications that complement the earlier volumes. There has been tremendous progress in the field in recent years, and this book puts together the most high-impact topics selected for their popularity by new editors Sergey Vyazovkin, Nobuyoshi Koga and Christoph Schick—all editors of Thermochimica Acta.
Among the important new techniques covered are biomass conversion; sustainable polymers; polymer nanocompsoties; nonmetallic glasses; phase change materials; propellants and explosives; applications to pharmaceuticals; processes in ceramics, metals, and alloys; ionic liquids; fast-scanning calorimetry, and more.
- Features 19 all-new chapters to bring readers up to date on the current status of the field
- Provides a broad overview of recent progress in the most popular techniques and applications
- Includes chapters authored by a recognized leader in each field and compiled by a new team of editors, each with at least 20 years of experience in the field of thermal analysis and calorimetry
- Enables applications across a wide range of modern materials, including polymers, metals, alloys, ceramics, energetics and pharmaceutics
- Overviews the current status of the field and summarizes recent progress in the most popular techniques and applications
Frequently asked questions
Information
Development of Direct and Indirect Methods for the Determination of Vaporization Enthalpies of Extremely Low-Volatile Compounds
† University of Bayreuth, Bayreut, Germany
Abstract
Keywords
1.1 Introduction
1.2 Kinetic Methods of Thermal Analysis (Vaporization) of Low-Volatile Compounds
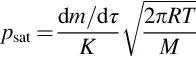
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributors
- Foreword
- Chapter 1: Development of Direct and Indirect Methods for the Determination of Vaporization Enthalpies of Extremely Low-Volatile Compounds
- Chapter 2: Fast Scanning Chip Calorimetry
- Chapter 3: Dilatometry
- Chapter 4: Modern Isoconversional Kinetics: From Misconceptions to Advances
- Chapter 5: Kinetics and Mechanisms of Solid-Gas Reactions
- Chapter 6: Physico-Geometric Approach to the Kinetics of Overlapping Solid-State Reactions
- Chapter 7: Analysis of Polymer Crystallization by Calorimetry
- Chapter 8: Glass Transition and Physical Aging of Confined Polymers Investigated by Calorimetric Techniques
- Chapter 9: Decomposition of Organic Wastes: Thermal Analysis and Evolution of Volatiles
- Chapter 10: Thermal Analysis of Biobased Polymers and Composites
- Chapter 11: Polymer Nanocomposites
- Chapter 12: Thermal Behavior of Chalcogenide Glasses
- Chapter 13: Applications of Thermal Analysis to the Study of Phase-Change Materials
- Chapter 14: Characteristics of Thermal Decomposition of Energetic Materials in a Study of Their Initiation Reactivity
- Chapter 15: Pharmaceutical Applications of Thermal Analysis
- Chapter 16: Thermoanalytical Characterization Techniques for Multiferroic Materials
- Chapter 17: Chalcogenides for Phase-Change Memory
- Chapter 18: Recent Advances in Thermal Analysis and Calorimetry of Aluminum Alloys
- Chapter 19: Metals and Alloys
- Index