
eBook - ePub
Clinical Trials
Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines
This is a test
- 896 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Clinical Trials
Study Design, Endpoints and Biomarkers, Drug Safety, and FDA and ICH Guidelines
Book details
Book preview
Table of contents
Citations
About This Book
Clinical Trials, Second Edition, offers those engaged in clinical trial design a valuable and practical guide. This book takes an integrated approach to incorporate biomedical science, laboratory data of human study, endpoint specification, legal and regulatory aspects and much more with the fundamentals of clinical trial design. It provides an overview of the design options along with the specific details of trial design and offers guidance on how to make appropriate choices. Full of numerous examples and now containing actual decisions from FDA reviewers to better inform trial design, the 2nd edition of Clinical Trials is a must-have resource for early and mid-career researchers and clinicians who design and conduct clinical trials.
- Contains new and fully revised material on key topics such as biostatistics, biomarkers, orphan drugs, biosimilars, drug regulations in Europe, drug safety, regulatory approval and more
- Extensively covers the "study schema" and related features of study design
- Incorporates laboratory data from studies on human patients to provide a concrete tool for understanding the concepts in the design and conduct of clinical trials
- Includes decisions made by FDA reviewers when granting approval of a drug as real world learning examples for readers
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Clinical Trials by Tom Brody in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Origins of Drugs
Abstract
A brief history of the discovery and invention of a selection of drugs is provided, as an introduction to this textbook on clinical trials. Examples were chosen from drugs that are classified as natural products, analogs of naturally occurring metabolites, antibodies, and drugs identified by screening libraries of chemicals. Also, as an introduction to clinical trials, information is given on scaling-up a drug dose suitable for animals, to that suitable for humans.
Keywords
Drug discovery; recombinant antibodies; animal models
I Introduction
Drugs have a number of origins, as outlined:
• Natural products, for example, chemicals from plants and microorganisms.
• Analogs of naturally occurring chemicals that reside in various biosynthetic pathways of mammals.
• Antibodies that bind to naturally occurring targets in the body.
• Discovery that an existing drug, established as effective for a first disease, is also effective for treating an unrelated second disease.
• Drugs identified by screening libraries of chemicals.
Some drugs are based on natural products, where the natural products were known to have pharmacological effects. The term “natural products” is a term of the art that generally refers to chemicals derived from plants, fungi, or microorganisms. Drugs that are derived from natural products, or that actually are natural products, include warfarin (1), penicillin (2,3), cyclosporine (4), aspirin (5,6), paclitaxel (7), fingolimod (8), and reserpine (9). Many other drugs have structures based on chemicals that occur naturally in the human body, that is, where the drugs are analogs of these chemicals. These include analogs of intermediates or final products of biosynthetic pathways. Drugs that are analogs of chemicals in biosynthetic pathways include methotrexate, cladribine, and ribavirin.
Still other drugs originated by first identifying a target cell, or target protein, and then by preparing antibodies that bind to that target. Vaccines have a similar origin. Once a target protein is identified, this target protein (or a derivative of it) can be formulated as a vaccine. Typically, vaccines take the form of the target protein derivative, called an “antigen,” in combination with a second compound that is an immune adjuvant.
Drugs are also derived using a screening assay and by testing hundreds or thousands of purified candidate compounds using that assay. Where the screening method is automated, the method is called high-throughput screening. The screening assay may consist of tumor cells that are cultured in vitro, where a robot determines if the candidate drug inhibits a particular enzyme in the tumor cell or if the candidate drug kills the tumor cell.
II Structures of Drugs
A knowledge of the structure of a drug to be used in a clinical trial is needed for the following reasons. First, the issue of whether a drug is hydrophobic or hydrophilic will dictate the nature of the excipient. If a drug is not water-soluble, then the excipient might need to include a solubilizing agent, such as a solvent. Second, the structure can also provide an idea of stability during long-term storage and thus in need of protection from light or in need of cold storage. Third, the structure can dictate the route of administration, and enable a prediction of pharmacokinetics of the drug and pathways of metabolism, transport, and excretion. Fourth, the structure of the drug can help the investigator predict adverse events that might be expected from the drug. For example, if the drug belongs to a class of compounds that activates cytochrome P450, some of the adverse events can be predicted. Fifth, regulatory submissions to the US Food and Drug Administration (FDA), such as the Investigational New Drug (IND), Investigator’s Brochure, and the package insert, typically contain a drawing of the drug structure.
a Origin of Warfarin
Warfarin is a drug that is widely used to prevent blood clotting, for example, in people at risk of heart attacks or strokes (10). A natural product produced during the spoiling of sweet clover inspired warfarin’s design. The drug was not named after any kind of warfare, even though it is used in warfare against mice and rats; it was named after the Wisconsin Alumni Research Foundation.
Spoiled sweet clover contains coumarin, a compound that inhibits an enzyme in the liver, where the end-result is impaired blood clotting. Blood clotting factors are biosynthesized in the liver, and then released into the bloodstream. Farmers in the mid-West found that cattle bled to death during the process of de-horning, where the cattle had eaten spoiled sweet clover. Eventually, one particular farmer in Wisconsin took a bucket of unclotted blood to researchers at the University of Wisconsin. The researchers examined the blood, as well as samples of spoiled sweet clover, and discovered that the culprit was dicoumarol, a degradative product of coumarin. Researchers synthesized and tested about 50 analogs of this compound. The analogs were tested in rabbits. It was discovered that the best analog was warfarin (11). Warfarin is also the active ingredient in rodent poison.
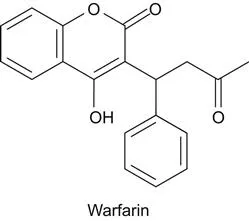
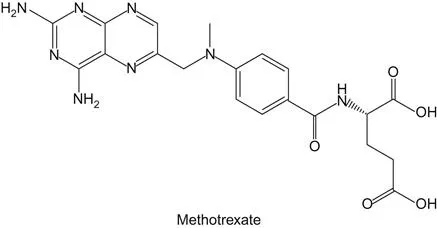
b Origins of Methotrexate and 5-Fluorouracil
The natural substrate of one particular enzyme, dihydrofolate reductase, inspired the design of methotrexate. This natural substrate is dihydrofolic acid (12). Dihydrofolic acid is the end-product of the biosynthetic pathway of folates (13). Anticancer drugs that inhibit dihydrofolate reductase were designed by synthesizing and screening chemicals that resembled dihydrofolate (14,15,16). Methotrexate, which is an analog of dihydrofolic acid, and also an analog of folic acid, inhibits dihydrofolic acid reductase. Another antifolate drug used in oncology is 5-fluorouracil. Fluorouracil was invented by Charles Heidelberger (17,18). The drug was developed on the basis of findings in the 1950s that cancer cells incorporated a larger amount of the uracil base into the DNA than normal cells. In testing a number of halogen-substituted uracil compounds, 5-fluorouracil appeared to be the most active and promising drug. Fluorouracil is a suicide inhibitor of thymidylate synthase. This means that the enzyme’s own catalytic activity results in the activation of the drug, where this activation causes the drug to react covalently with the enzyme, thereby destroying the enzyme’s catalytic activity.
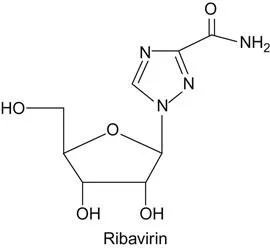
c Origin of Ribavirin
Ribavirin was discovered by synthesizing analogs of a compound participating in the pathways of nucleotide biosynthesis. In designing, synthesizing, and testing a variety of analogs of intermediates in nucleotide biosynthetic pathways, the result was the discovery of ribavirin, also known as virazole (19,20).
Ribavirin, in combination with one or more drugs, is used to treat HCV infections (21,22,23,24,25,26,27,28,29). The other drugs in this combination include sofosbuvir, dasabuvir, and pegylated interferon-alfa. Ribavirin alone has been used to treat hepatitis E virus (30).
d Origin of Paclitaxel
Paclitaxel (Taxol®), an anticancer drug, was discovered in extracts of the Pacific yew tree, Taxus brevifolia. In 1963, a crude extract from Pacific yew bark was found to have activity against tumors in experimental animals (31). In 1991, the active component, paclitaxel, was approved by the FDA as an anticancer drug. Paclitaxel, which is a class of drugs called taxanes, acts on the cytoskeleton of the cell. Specifically, the drug acts on tubulin, disrupts the normal behavior of the cytoskeleton in mediating cell division, and causes cell death (32). Docetaxel (Taxotere®) is a semisynthetic analog of paclitaxel (33) having a mechanism and anticancer properties similar to those of paclitaxel. Docetaxel can be synthesized using a precursor extracted from needles of the European yew, Taxus baccata (34). Paclitaxel ...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Acknowledgments
- Preface
- Introduction
- Abbreviations and Definitions
- Biographies
- Chapter 1. Origins of Drugs
- Chapter 2. Clinical Trial Design
- Chapter 3. Run-In Period
- Chapter 4. Inclusion/Exclusion Criteria, Stratification, and Subgroups—Part I
- Chapter 5. Inclusion/Exclusion Criteria, Stratification, and Subgroups—Part II
- Chapter 6. Blinding, Randomization, and Allocation
- Chapter 7. Placebo Arm as Part of Clinical Trial Design
- Chapter 8. Intent-to-Treat Analysis Versus Per Protocol Analysis
- Chapter 9. Biostatistics—Part I
- Chapter 10. Biostatistics—Part II
- Chapter 11. Introduction to Endpoints
- Chapter 12. Oncology Endpoint—Objective Response
- Chapter 13. Oncology Endpoints: Overall Survival and Progression-Free Survival
- Chapter 14. Oncology Endpoints: Time to Progression
- Chapter 15. Oncology Endpoint: Disease-Free Survival
- Chapter 16. Oncology Endpoint: Time to Distant Metastasis
- Chapter 17. Neoadjuvant Therapy Versus Adjuvant Therapy
- Chapter 18. Hematological Cancers
- Chapter 19. Biomarkers
- Chapter 20. Endpoints for Immune Diseases
- Chapter 21. Endpoints for Infections
- Chapter 22. Health-Related Quality of Life Tools—Oncology
- Chapter 23. Health-Related Quality-of-Life Tools—Immune Disorders
- Chapter 24. Health-Related Quality-of-Life Tools—Infections
- Chapter 25. Drug Safety
- Chapter 26. Mechanism of Action of Diseases and Drugs—Part I
- Chapter 27. Mechanism of Action—Part II (Cancer)
- Chapter 28. Mechanism of Action—Part III (Immune Disorders)
- Chapter 29. Mechanisms of Action—Part IV (Infections)
- Chapter 30. Consent Forms
- Chapter 31. Package Inserts
- Chapter 32. Warning Letters
- Chapter 33. Regulatory Approval
- Chapter 34. Patents
- Index
- Sync with Jellybooks