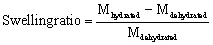Abstract:
The ability of hydrogels to respond to relatively small changes in stimuli with relatively large changes in volume allows a wide variety of applications. This chapter addresses hydrogels with regard to the chemical identity of hydrophilic polymers and copolymers, polymer synthesis, the degree of crosslinking and hydrogel porosity, and bulk geometry of hydrogels in the form of matrix, membrane and erodible systems. The relationships between these features and hydrogel swelling behavior upon stimulation are also described. Finally, various exploitations of hydrogel swelling behavior in developing highly sensitive, real-time biosensors are discussed.
1.1 Basics of hydrogels
Hydrogels gained increased attention from the scientific community in the latter half of the 20th century (Brannon-Peppas and Peppas, 1991). The ability of hydrogels to respond to relatively small changes in external stimuli with relatively large changes in bulk volume enables direct detection of a variety of stimuli (Gemeinhart et al, 2000; Lee and Park, 1996; Peppas et al., 2000; Roy and Gupta, 2003). The chemical makeup, synthesis, crosslinking, and geometry of hydrogels are briefly described.
1.1.1 Chemical identity of hydrophilic polymers and copolymers
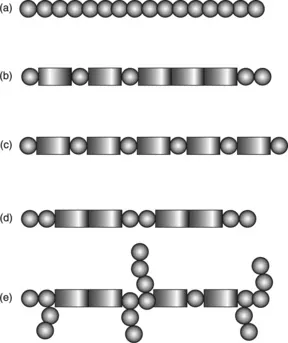
Hydrogels are composed of hydrophilic polymer chains. These chains may consist of repeating monomers (homopolymers) or chemically different monomers (copolymers) (Peppas et al., 2000). As depicted in Fig. 1.1, monomers can be arranged in such a way to make random copolymers, alternating copolymers, block copolymers, or graft copolymers. Additionally, the polymer chains may form more intricate three-dimensional structures, such as five-pointed star polymers, or dendrimers (Jeong et al., 2002). The selection of chemical makeup of a polymer is critical to controlling swelling behavior, since these constituents are responsible for interactions with water and subsequent volume change (Peppas et al., 2000). For example, hydrogels with hydrophobic internal cores would be well suited for delivery of poorly water-soluble drugs (Jeong et al., 2002).
1.1 Chemical diversity of hydrogel polymer chains. (a) homopolymer, (b) random copolymer, (c) alternating copolymer, (d) block copolymer, and (e) graft copolymers. Rectangular and circular units represent chemically different monomers.
1.1.2 Polymer synthesis
Polymer synthesis is tailored according to the need to develop chemically diverse hydrogels for specific applications (Brannon-Peppas and Peppas, 1991; Peppas et al., 2000). Depending on the application, the synthesized polymers may require biocompatibility, mechanical strength, or analyte specificity in addition to sensitivity to stimuli (Brannon-Peppas and Peppas, 1991; Lee and Park, 1996; Peppas et al, 2000; Kim and Park, 2001b; Kim and Park, 2004). Table 1.1 lists monomers used in synthesizing hydrogels for pharmaceutical applications. Polymers are synthesized by various mechanisms, such as radical polymerization, condensation polymerization, graft-copolymerization, photopolymerization, and ring-opening polymerization (Lee and Park, 1996; Kim and Park, 2001b; Lee et al, 2003; Xiao, 2007; Pearton et al, 2008; Xue et al, 2004; Plunkett et al, 2003; Gu et al., 2002). Care must be taken to purify the synthesized hydrogels for pharmaceutical and biomedical applications by removing the residual monomer, initiator, crosslinking agent and other contaminants (Markowitz et al., 1997; Risbud et al., 2000; Peppas et al., 2000).
Table 1.1
Examples of monomers used in pharmaceutical applications
Monomer
Acrylic acid (AA)
Ethylene glycol (EG)
Hydroxyethyl methacrylate (HEMA)
N-isopropyl acrylamide (NIPAAm)
N-vinyl-2-pyrrolidone (NVP)
Poly(ethylene glycol acrylate) (PEGA)
Source: Peppas et al. (2000)
1.1.3 Degree of crosslinking and porosity
Hydrogels are crosslinked either physically or chemically to form networks (Peppas et al., 2000; Roy and Gupta, 2003). Physical crosslinking occurs via noncovalent interactions, whereas chemical crosslinking utilizes covalent interactions (Lin et al., 2005). The degree of crosslinking plays a significant role in the integrity and swelling properties of hydrogels, influencing hydrogel structure and swelling capacity (Flory and Rehner, 1943; Brannon-Peppas and Peppas, 1991). The greater the extent of crosslinking, the less flexible a hydrogel is to shrink, swell or change phase in response to stimuli (Peppas et al., 2000). Hydrogel brittleness has been observed at high degrees of crosslinking (Peppas et al., 2000). Physical crosslinks are often used in hydrogel formation due to their ability to reform crosslinks upon removal or presentation of the stimulus (Roy and Gupta, 2003; Lee and Park, 1996; Lee et al., 2004).
Hydrogels have a range of porosities that influence the diffusion coefficients involved in mass transfer during swelling (Peppas et al., 2000; Bezemer et al., 2000). Pore size is dependent on the average molecular weight of polymer chain segments between adjacent crosslinks and acts as a selective barrier with regard to the permeability of substances (Peppas et al., 2000). Specifically, swelling can be decreased by decreasing the average molecular weight of the polymer chain segments between crosslinks (Brannon-Peppas and Peppas, 1991). Pore size can be further controlled by various techniques, such as freeze drying, porosigen method, or gas formation method (Gemeinhart et al., 2000). Therefore, the pores can range from a few nanometers to several micrometers (Kim and Park, 2004).
1.1.4 Bulk geometry of hydrogels
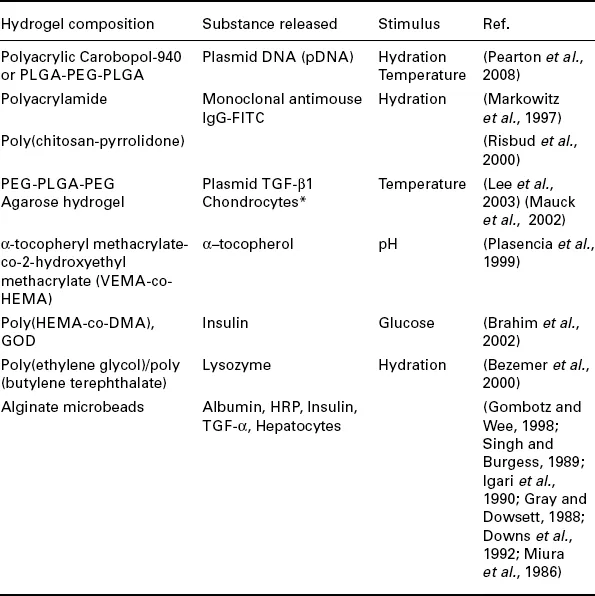
Hydrogels can be molded into various geometries, ranging from microspheres to films, and this makes their application highly versatile (Roy and Gupta, 2003). Hydrogel matrixes can be used as implantable scaffolds, due to their structural properties and ability to absorb or release bioactive substances (Roy and Gupta, 2003; Pearton et al., 2008; Markowitz et al, 1997; Risbud et al, 2000; Lee et al, 2003; Mauck et al, 2002; Gombotz and Wee, 1998). Table 1.2 lists hydrogels that have been studied for controlling the release of bioactive substances. Due to the relative thickness of a hydrogel matrix (as compared to membranes), the rate of diffusion for drug molecules through the matrix may be impeded (Zhang and Wu, 2002). Conversely, hydrogel membranes are relatively thin and offer increased response rate (swelling or shrinking) to stimuli due to the shorter distance required for diffusion (Zhang and Wu, 2002). Such hydrogels, capable of preventing degradation of labile substances, act as their reservoirs until stimulated (Pearton et al., 2008; Markowitz et al, 1997; Risbud et al, 2000; Mauck et al, 2002; Bezemer et al, 2000). Similarly, erodible hydrogels are of interest in the pharmaceutical field as their ability to exhibit zero-order release kinetics has been well established (Peppas et al., 2000; Lee, 1984).
Table 1.2
Examples of the use of hydrogels in biomedical applications
Note:
*seeded in hydrogel
1.2 Swelling of hydrogels: water diffusion into hydrogels
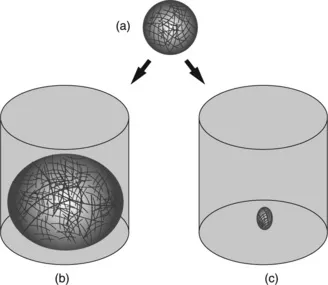
The ability to display a measurable change in volume in response to external stimuli is a fundamental property of hydrogels (Lee and Park, 1996). Some hydrogels exhibit this volume change by swelling (see Fig. 1.2), while others undergo transitions between sol and gel phases (Brannon-Peppas and Peppas, 1991; Gemeinhart et al, 2000; Lee and Park, 1996; Jeong et al, 2002). When hydrogels swell, the glassy phase turns into the rubbery phase (Lee, 1984). The degree of crosslinking influences the area permitted for diffusion across the hydrogel network and, subsequently, the capacity for hydrogels to take up water (Peppas et al., 2000). The water capacity is depicted from the equilibrium swelling ratio shown in Equation 1.1, as the ratio of the mass of a fully swollen hydrogel (in equilibrium with aqueous medium) to the mass of a dehydrated hydrogel (Brannon-Peppas and Peppas, 1991).
1.2 Dehydrated (a), swollen (b), and shrunken (c) hydrogels as the result of small changes in external stimuli, such as pH, temperature and analyte concentration that influence hydrogel hydrophilicity.
where M represents hydrogel mass. Interactions between polymers in hydrogels and water are similar in nature to those between non-cros...