![]()
THE NITROGEN ATOM
Publisher Summary
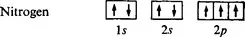
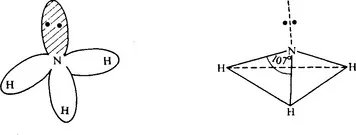
Nitrogen has atomic number 7 and the structure ls2 2s2 2p3. The three 2p electrons are disposed singly in the 2px, 2py, and 2pz orbitals. The nitrogen atom may form three covalent bonds with three hydrogen atoms resulting in stable ammonia molecule, NH3, in which the nitrogen atom still owns a lone pair of electrons.
NITROGEN having atomic number 7 has the structure 1s22s22p3, the three 2p electrons disposed singly in the 2px, 2py and 2pz orbitals. The ground state of a nitrogen atom is represented as follows:
It is therefore not surprising that the nitrogen atom may form three covalent bonds with each of three hydrogen atoms with the resulting stable ammonia molecule NH3 in which the nitrogen atom still owns a lone pair of electrons.
![]()
TYPES OF AMINES
Publisher Summary
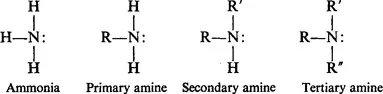
This chapter discusses types of amines. Amines are the derivatives of ammonia in which one or more hydrogen atoms have been replaced by carbon-containing groups. It does not mean that the only way of preparing amines from ammonia is by the substitution of the hydrogen atoms by carbon-containing groups. The chapter discusses different methods of preparing amines and in those methods, ammonia is not one of the reactants. Any organic substance in which the —NH2 group is present is called a primary amine; this group is attached to a carbon-containing group R that may be as simple as CH3 or quite a bit more complex in structure. An organic substance that contains an >NH group attached to two carbon-containing groups of varying complexity is called a secondary amine, and one in which three carbon-containing groups are attached to a nitrogen atom is called a tertiary amine. In all the cases, the lone pair of electrons is present on the respective nitrogen atoms.
We can consider various organic compounds containing a nitrogen atom at the same oxidation level as in ammonia, as derivatives of ammonia in which, formally, one or more hydrogen atoms have been replaced by carbon-containing groups. Because of this way of looking at these derivatives these substances have been given the name amines.
This formal representation is useful only insofar as it emphasizes the relationship of the various classes of amines to ammonia; it does not mean necessarily that the only way of preparing amines is by substitution of the hydrogen atoms in ammonia by carbon-containing groups. Quite the contrary, we shall discuss many methods of preparing amines in which ammonia is not one of the reactants.
The above four formulae show that any organic substance in which the —NH2 group is present is called a primary amine; this group is attached to a carbon-containing group R which may be as simple as CH3 or quite a bit more complex in structure. Whatever the case, it should be noted that the nitrogen atom still owns, of itself, a lone pair of electrons. (This last statement will be somewhat qualified in our later discussion.)
An organic substance which contains an 〉NH group attached to two carbon-containing groups of varying complexity is called a secondary amine and one in which three carbon-containing groups are attached to a nitrogen atom is called a tertiary amine. Here also it should be emphasized that the lone pair of electrons is present on the respective nitrogen atoms.
![]()
BASICITY OF AMINES
Publisher Summary
Amines are important bases of organic chemistry. This chapter discusses the basicity of amines. It is the lone pair on nitrogen atom that confers the basic character of the amines. If the delocalization of the lone pair occurs through its involvement with other parts of the molecule because of resonance, the basicity of the amine may be low indeed. The chapter illustrates the ionization of primary amines in aqueous solution. When ammonia reacts with a proton to give an ammonium ion, the ammonium ion must have a single positive charge to obey the law of conservation of energy. A proton is a hydrogen atom that has lost its single electron or single unit of negative charge and therefore, it is positively charged. The nitrogen atom contributes its lone pair to form a two-electron covalent bond between it and the proton. Once this has happened, one cannot tell the difference between the three nitrogen–hydrogen bonds already existing in ammonia and the fourth newly-created nitrogen–hydrogen bond.
The presence of the lone pair cannot be overemphasized since it is the lone pair which confers upon the amines, whether they be primary, secondary or tertiary, their basic character. The amines are the important bases of organic chemistry. They may be stronger or weaker bases but the fact that they are bases is due to the lone pair on nitrogen. If delocalization of the lone pair occurs through its involvement with other parts of the molecule due to resonance, the basicity of the amine may be very low indeed and we shall exemplify this in our discussion of certain aromatic and heterocyclic amines. However, in principle, the basicity of the amines ma...