
- 491 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Nucleic Acid Nanotheranostics: Biomedical Applications offers a comprehensive overview of improvements and new trends in fabrication of nanostructures as theranostic multifunctional carriers in gene therapy. With a strong focus on medical applications (comprising diagnosis, therapy and imaging), the book also examines gene therapy in an individual patient's cells or tissues to treat genetic diseases. Sections cover Biomedical and Diagnostic applications of Nucleic Acids, Biologic and Synthetic Advanced Nanostructures for nucleic acid delivery, and important considerations of nanomedicine. This book is a valuable guide for materials scientists, physicians, chemists and engineers, but is also ideal for clinicians wishing to expand their knowledge.
- Provides a unique source of knowledge (theoretical as well as practical) on nanotheranostic materials for gene therapy at all levels and related scientific areas
- Covers the pros and cons related to viral and nanomaterial-based delivery of nucleic acids in terms of biosafety, carrier selection, synthesis and bioimaging
- Presents the only book to include an analysis of nanoformulations approved for clinical use
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Therapeutic Oligonucleotides Against Cancer: Recent Approaches and New Perspectives
Giovanni Palomino-Vizcaino; Luis M. Alvarez-Salas Laboratory of Gene Therapy, Department of Genetics and Molecular Biology, Centro de Investigación y de Estudios Avanzados del I.P.N., Mexico City, Mexico
Abstract
The use of therapeutic oligonucleotides (TONs) has become a major alternative to treat disease thanks to recent advances in oligonucleotide synthesis and delivery technologies. Cancer is the result of progressive and accumulative changes in the cell genome and the host physiology that lead to uncontrolled growth and proliferation, metabolic changes, immune evasion, and ultimately metastasis and death. The present review focuses on oligonucleotide-based cancer therapies that have reached clinical trials. Although very effective in cell culture, TONs have faced severe delivery, pharmacokinetic, and stability issues. New conjugate, encapsulation, and formulation technologies improved the clinical application of TONs to treat cancer. Moreover, improvements in biostability and delivery have generated expectancies in cancer vaccine development, immunotherapy, and early detection. Therefore, the specificity, structural diversity, and versatility showed by TONs promise that they will become the treatment of choice for several types of cancer in the next few years.
Keywords
Oligonucleotides; Theranostics; Therapy; Cancer; Cancer therapy; Antisense; Catalytic oligonucleotides; Ribozymes; DNAzymes; siRNA; miRNA; Aptamers; DNA; RNA; Delivery
1 Introduction
In the last decade, cancer has been broadly accepted as an organismal disease in which a tumor can feed, expand, and invade. Conceptually, a tumor is a complex tissue composed of multiple cell types that interact with each other to provide a microenvironment in which abnormal cells that have lost control over their proliferation and lifespan can multiply and evade the immune response leading to metastasis. A tumor cell undergoes several genetic changes that allow for sustained, aberrant, and undefined proliferation, apoptosis inhibition, angiogenesis induction, and activation of migration and invasion. Such changes, the hallmarks of cancer, result in tumor cell genomic instability leading to genetic diversity, modifications in energy metabolism, inflammation and immune evasion. Therefore, therapeutic approaches to cancer must affect one or more hallmark capabilities thus impairing tumor growth and progression.1
The unique biological and physicochemical properties of oligonucleotides (ONs) appear very suitable to fight cancer. ONs can be easily engineered to block the flow of genetic information at all levels (replication, transcription, translation, and recombination). Thus, ONs can be directed to block the expression of specific targets involved in cell proliferation, apoptosis inhibition, cell cycle progression, cell migration, and invasion. Because of their remarkable flexibility and adaptive structure, ONs can also be used to specifically recognize and bind target molecules with high affinities, inhibiting functions, such as receptor-ligand interactions, or stimulating immune responses.
The use of ONs as therapeutic agents dates back over half a century, with the initial usage of nucleoside analogues to fight disease. Nowadays, therapeutic ONs (TONs) are applied to treat a wide range of pathological and infection-related conditions and are readily available worldwide.2 Advances in nucleic acid chemical synthesis allowed for the affordable production of short (< 100 nt) single-stranded DNA or RNA ONs that, in conjunction with the wide availability of genomic data and genetic engineering technologies, resulted in the rationalized development of promising bioinformatics-based strategies for cancer detection and treatment. TONs have raised high expectations that have been obscured by the frequent unexpected effects found in failed treatments and clinical trials. A sound experimental background and laboratory testing is now required before proposing TONs as the “magic bullets” to cure disease.
As with other drugs, the success of ONs as therapeutic moieties is determined both by their capacity to affect a specific target (sequence complementarity with the target, activation of intracellular mechanisms, such as RNA interference, immunogenicity, and others) and their pharmacokinetic performance (absorption, distribution, metabolism, and excretion).3 From their interaction with the target, or pharmacophore, TONs-based therapy for cancer can be classified into five main technologies with different principles of action: (1) antisense ONs (AS-ODNs), which hybridize to and translationally silence a target mRNA; (2) RNA interference (RNAi) moieties (siRNAs and miRNAs), with the capacity to hybridize the target mRNA and induce silencing through RNA interference (RNAi); (3) immunomodulatory ONs (IMOs) that induce innate immune response; (4) catalytic ONs (DNAzymes and Ribozymes) able to hybridize and cleave a target mRNA; and (5) aptamers, which are ONs able to structurally recognize and bind target molecules. All of these technologies have been thoroughly tested in vitro and have found clinical applications in cancer therapy.4
From the pharmacokinetic point of view, TONs require the elicitation of either potent tissue-specific responses (gene-silencing or modulation, protein interaction, etc.) or systemic responses (immunostimulatory or immunosuppressing) following a hierarchical distribution pattern.3 After application (systemic or local), TONs are rapidly absorbed and widely distributed through the whole organism, although most of them end up in the liver and kidneys, bone marrow, and lymph nodes.5 Once in the target organ or tissue, TONs are taken up by the cell and transported to the intracellular site of action (cell membrane, nucleus, organelles, or cytoplasm) to meet their respective targets and exert their function.
The informational nature of TONs-based therapies in the postgenomic era has suggested the accelerated rational design of drugs against virtually any genetic target. However, although many TONs-based therapies have been developed, not a single TON technology has yet been approved for cancer therapy.6 It is now clear that a profound knowledge of the pharmacokinetics and pharmacodynamics properties of each technology is required to develop successful TONs. Because of the huge amount of information on this subject, we will focus only on those TONs that have reached clinical trials with anticancer protocols.
2 Mechanisms of Action
2.1 Antisense Oligodeoxynucleotides (AS-ODNs)
The concept of antisense therapy was originally developed by Stephenson and Zamecnik when they showed the ability of an antisense oligodeoxynucleotide (AS-ODN) to inhibit RNA translation of the Rous sarcoma virus (RoSV).7 AS-ODNs use sequence data from a target gene to synthesize ONs complementary to their mRNA, impeding translation either by inducing the degradation of the target mRNA (RNaseH activation) or by blocking ribosome binding or elongation (Fig. 1).8 AS-ODNs were initially identified as a promising strategy for the design of specific gene-silencing drugs.9,10 Although AS-ODNs are easily delivered to cells, they are highly unstable in biofluids, requiring chemical modifications that produce considerable (and sometimes fatal) toxicity and that may trigger uncontrolled immunological effects on the target cells (off-target effects).
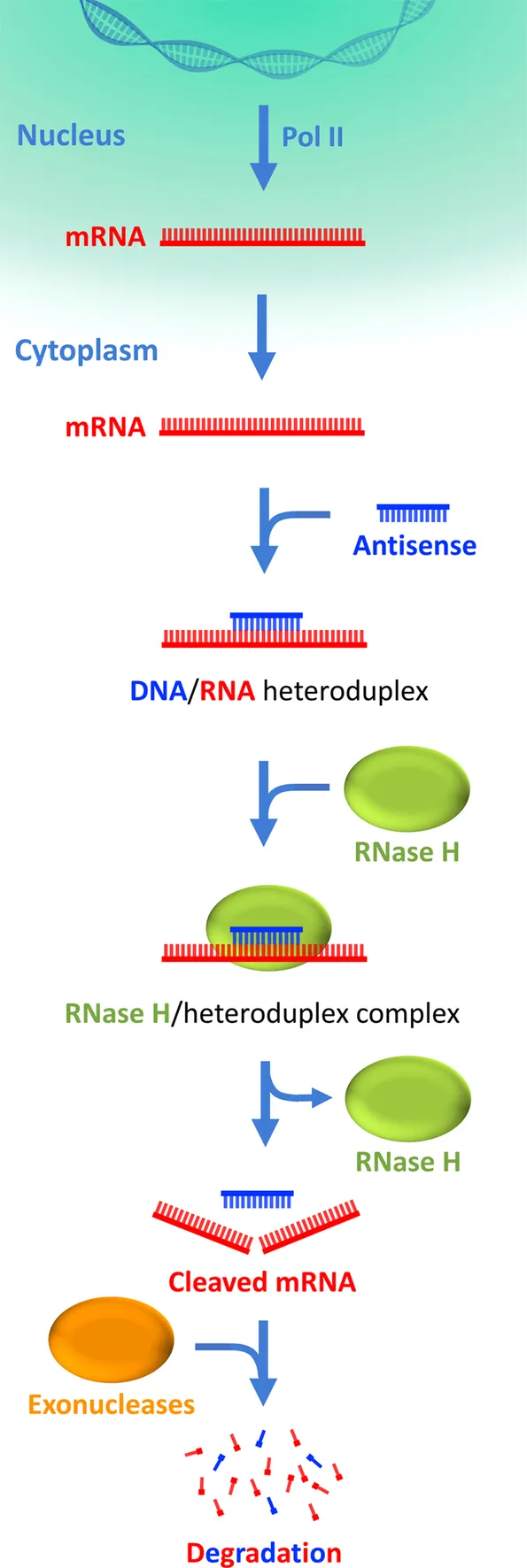
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Contributors
- Preface
- Acknowledgments
- Chapter 1: Therapeutic Oligonucleotides Against Cancer: Recent Approaches and New Perspectives
- Chapter 2: Small Interfering RNA-Mediated Silencing of the Ribophorin II Gene: Advances in the Treatment of Malignant Breast Cancer
- Chapter 3: Noncoding RNAs in Cardiovascular Disease
- Chapter 4: MicroRNAs in Respiratory Diseases
- Chapter 5: State of the Art and Future Direction for the Analysis of Cell-Free Circulating DNA
- Chapter 6: Aptamer-Based Strategies for Diagnostics
- Chapter 7: Viral Vectors for Treatment of Human Disease: Therapeutic and Manufacturing Challenges
- Chapter 8: Nonviral Gene Therapy: Peptiplexes
- Chapter 9: Controlling Protein Expression by Delivery of RNA Therapeutics Using Lipid Nanoparticles
- Chapter 10: Advanced Polymers for Nonviral Gene Delivery
- Chapter 11: Nonviral Gene Therapy: Design and Application of Inorganic Nanoplexes
- Chapter 12: Extracellular Vesicles as Novel Nanocarriers for Therapeutic Delivery
- Chapter 13: Nanocarrier-Based Gene Therapy Imaging Strategies
- Chapter 14: The State of the Art of Investigational and Approved Nanomedicine Products for Nucleic Acid Delivery
- Conclusions and Future Outlook
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Nucleic Acid Nanotheranostics by Marco Filice,Jesús Ruiz-Cabello in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Biotechnology in Medicine. We have over one million books available in our catalogue for you to explore.