
eBook - ePub
Intelligent Coatings for Corrosion Control
Atul Tiwari,Lloyd Hihara,James Rawlins
This is a test
Share book
- 746 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Intelligent Coatings for Corrosion Control
Atul Tiwari,Lloyd Hihara,James Rawlins
Book details
Book preview
Table of contents
Citations
About This Book
Intelligent Coatings for Corrosion Control covers the most current and comprehensive information on the emerging field of intelligent coatings. The book begins with a fundamental discussion of corrosion and corrosion protection through coatings, setting the stage for deeper discussion of the various types of smart coatings currently in use and in development, outlining their methods of synthesis and characterization, and their applications in a variety of corrosion settings. Further chapters provide insight into the ongoing research, current trends, and technical challenges in this rapidly progressing field.
- Reviews fundamentals of corrosion and coatings for corrosion control before delving into a discussion of intelligent coatings—useful for researchers and grad students new to the subject
- Covers the most current developments in intelligent coatings for corrosion control as presented by top researchers in the field
- Includes many examples of current and potential applications of smart coatings to a variety of corrosion problems
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Intelligent Coatings for Corrosion Control an online PDF/ePUB?
Yes, you can access Intelligent Coatings for Corrosion Control by Atul Tiwari,Lloyd Hihara,James Rawlins in PDF and/or ePUB format, as well as other popular books in Naturwissenschaften & Industrielle & technische Chemie. We have over one million books available in our catalogue for you to explore.
Information
Topic
NaturwissenschaftenSubtopic
Industrielle & technische ChemieChapter 1
Electrochemical Aspects of Corrosion-Control Coatings
L.H. Hihara Hawaii Corrosion Laboratory, Department of Mechanical Engineering, University of Hawaii at Manoa, Honolulu, Hawaii, USA
Abstract
Corrosion of metals is an electrochemical process and is affected by moisture content, the chemical environment, and the electrochemical state of the metal. How the properties of a coating or coating system affect the above parameters governs the effectiveness of the coating in suppressing corrosion. Fundamental electrochemical aspects of corrosion are discussed, as well how coating properties (i.e., barrier characteristics and electrical resistivity, chemical and electrochemical) affect the corrosion behavior of the substrate metal. Barrier protection, corrosion inhibition, and cathodic protection as they relate to coatings are discussed.
Keywords
Corrosion protection
Coatings
Barrier
Corrosion inhibition
Cathodic protection
1.1 Introduction
In nature, almost all metals are found in their thermodynamically stable states which are ores that are comprised primarily of oxides, sulfides, and halides.1 Energy must be expended to extract the elemental metals from the ores. Hence, as soon as the elemental metals are extracted from their ores, they have a propensity to revert back to their thermodynamically stable compounds. In most cases, metals will form oxides when exposed to moisture. If the oxide is porous or does not have good adhesion to the substrate metal, the metal will actively corrode. If the oxide forms a compact impervious layer and has good adhesion, the metal will passivate, resulting in excellent corrosion resistance. In environments that contain aggressive ions, however, the passive film can breakdown, resulting in localized corrosion and very high corrosion rates. Hence, coatings are very frequently needed to suppress corrosion in metals that do not naturally form protective passive films or for aggressive environments that can break down passivity.
1.2 Corrosion
For a metal to corrode by aqueous corrosion, water molecules must be present. The metal, however, need not be fully immersed because water can condense on a surface due to temperature fluctuations, even if the relative humidity is lower than 100% (e.g., condensation on cold surface on a warm, humid day), or due to hygroscopic impurities such as airborne salts.2
1.2.1 Thermodynamics
Aqueous corrosion is an electrochemical process involving anodic (or oxidation) and cathodic (or reduction) reactions. Dissolution of a metal M, which is an anodic reaction, is represented by the half-cell reaction
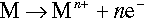
The electrons of the anodic reaction must be consumed by a cathodic reaction for corrosion to proceed. Two predominant cathodic reactions in aqueous corrosion are oxygen reduction (Equations 1.2 and 1.3) and hydrogen evolution (Equations 1.4 and 1.5), respectively. Their half-cell reactions are represented as follows:
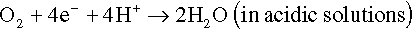
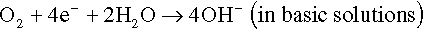
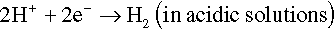
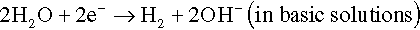
Oxygen reduction can only occur in aerated solutions, which contain dissolved oxygen molecules. Hydrogen evolution can occur ...