
Compendium of Hydrogen Energy
Hydrogen Energy Conversion
- 328 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Compendium of Hydrogen Energy
Hydrogen Energy Conversion
About This Book
Compendium of Hydrogen Energy: Hydrogen Energy Conversion, Volume Three is the third part of a four volume series and focuses on the methods of converting stored hydrogen into useful energy. The other three volumes focus on hydrogen production and purification; hydrogen storage and transmission; and hydrogen use, safety, and the hydrogen economy, respectively.
Many experts believe that, in time, the hydrogen economy will replace the fossil fuel economy as the primary source of energy. Once hydrogen has been produced and stored, it can then be converted via fuel cells or internal combustion engines into useful energy.
This volume highlights how different fuel cells and hydrogen-fueled combustion engines and turbines work. The first part of the volume investigates various types of hydrogen fuel cells, including solid oxide, molten carbonate, and proton exchange membrane. The second part looks at hydrogen combustion energy, and the final section explores the use of metal hydrides in hydrogen energy conversion.
- Highlights how different fuel cells and hydrogen-fueled combustion engines and turbines work
- Features input written by leading academics in the field of sustainable energy and experts from the world of industry
- Examines various types of hydrogen fuel cells, including solid oxide, molten carbonate, and proton exchange membrane
- Presents part of a very comprehensive compendium which, across four volumes, looks at the entirety of the hydrogen energy economy
Frequently asked questions
Information
Proton exchange membrane fuel cells
† Université de Picardie Jules Verne, Amiens Cedex, France
‡ Réseau sur le Stockage Electrochimique de l’Energie (RS2E), Amiens, France
§ ALISTORE European Research Institute, Amiens Cedex, France
Abstract
1.1 Fabrication and manufacturing of fuel cells
1.1.1 Introduction
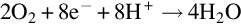
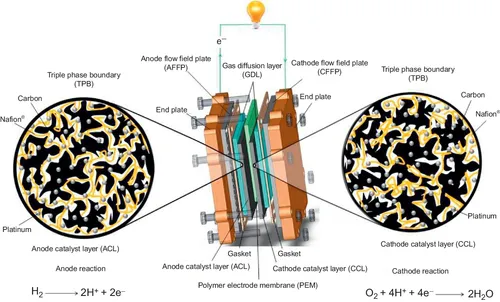
1.1.2 Principles of membrane electrode assembly
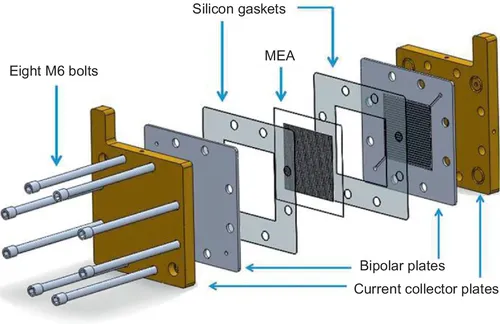
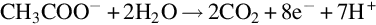
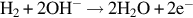
1.1.3 Overview of MEA components
1.1.3.1 Catalyst layer
1.1.3.2 Gas diffusion layer
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- List of contributors
- Woodhead Publishing Series in Energy
- Part One: Fuel cells
- Part Two: Hydrogen combustion and metal hydride batteries
- Index
- Sync with Jellybooks