eBook - ePub
Cancer Treatment and the Ovary
Clinical and Laboratory Analysis of Ovarian Toxicity
This is a test
- 166 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Cancer Treatment and the Ovary
Clinical and Laboratory Analysis of Ovarian Toxicity
Book details
Book preview
Table of contents
Citations
About This Book
Cancer Treatment and the Ovary: Clinical and Laboratory Analysis of Ovarian Toxicity provides the reader with a basic understanding on how the ovary is adversely impacted by cancer treatment, an essential foundational knowledge for this rapidly-developing field.
The book describes both the clinical and laboratory approaches to discovering the potentially adverse effects of cancer treatment on the ovary, also laying out possible preventative approaches and future directions for the field.
Clinicians working in the field of reproductive biology and oncology will find an essential reference that provides the necessary tools to assess the reproductive toxicological effects of cancer treatments.
- Brings together an international group of experts to address the current state of the science of ovarian toxicity caused by cancer treatment
- Provides scientific, clinical, and preclinical approaches to assessing this toxicity
- Describes current techniques and future strategies to protect the ovary
- Ideal reference for the further study of ovarian toxicity, oncofertility, cancer treatment, and reproductive toxicology
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Cancer Treatment and the Ovary by Richard A Anderson,Norah Spears in PDF and/or ePUB format, as well as other popular books in Medicine & Oncology. We have over one million books available in our catalogue for you to explore.
Information
Section III
Strategies to Protect the Ovary
Outline
Chapter 7
Ovarian Tissue Cryopreservation for Fertility Preservation
Annette Klüver Jensen, Stine Gry Kristensen and Claus Yding Andersen, Laboratory of Reproductive Biology, Copenhagen University Hospital – Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
Cryopreservation and transplantation of ovarian tissue is the only option available for preserving the fertility of prepubertal girls and for women who need acute gonadotoxic cancer treatment. To date, 39 healthy children have been born worldwide using this technique and it is becoming well established in many countries. However, the long-term success rate is difficult to determine since both the numerator and the denominator are difficult to define. A pregnancy may ensue as long as the tissue is active but the true efficacy cannot be determined until the tissue has stopped working. Further, some women wish to become pregnant while others wish only to regain hormone function. The longevity of ovarian grafts and thereby a woman’s fertility potential depends on the initial follicle pool, which is related, among other factors, to age, cryo injury and the time it takes post-transplantation until the vascular beds have been revascularized.
A major concern for this procedure is the risk of transplanting malignant cells, which could be inherent in the grafted tissue. It is estimated that worldwide over 150 transplantations (October 2014) have been performed and the vast majority of these cases have restored ovarian function/activity; some have lasted over 7 years, with no relapses at the graft sites so far.
Keywords
autotransplantation; fertility preservation; malignant cell contamination; outcome; ovarian tissue cryopreservation
7.1 Overview
The survival rate among the ~2% of women of reproductive age who have suffered from invasive cancer has substantially increased.1 Unfortunately, the necessary chemo- and radiotherapy carry the risk of unwanted side effects such as permanent infertility,2 jeopardizing a woman’s chances of having her own biological children.
Until recently, in vitro fertilization (IVF) and embryo transfer (ET) in combination with cryopreservation were considered the only options for women to conceive after recovery from a sterilizing cancer treatment. However, these methods carry some drawbacks and cannot sustain long-term fertility. They require controlled ovarian stimulation, and are not applicable to prepubertal girls. Cryopreservation and transplantation of ovarian tissue fulfils a number of these shortcomings.3,4 Grafting of cryopreserved ovaria.;n tissue can restore menstrual cyclicity, does not require pretreatment and can be performed without delay. Further, the method does not require male gametes and is applicable even to prepubertal girls.
It is very difficult to give a patient an estimation of the risk of premature ovarian insufficiency (POI) due to the number of contributory factors, including age, disease, stage of disease and the fact that the planned chemotherapy treatment often changes during the course of treatment.5 To help the physicians evaluate each patient, selection criteria such as the Edinburgh criteria (Figure 7.1)6 can be used for guidance. However, these criteria should merely be used as guidance and not exact guidelines, as each woman should have an individual medical assessment of her ovarian reserve as well as her risk of POI. At the same time, she should have the opportunity to decide whether she is willing to take the risk of the operation in relation to the risk of becoming infertile. In Denmark, there is no exact upper age limit for women having ovarian tissue cryopreserved because some women have a better fertility potential than others (e.g., a high number of antral follicles) despite increased biological age (>35 years of age) and would therefore still benefit from having ovarian tissue cryopreserved. Furthermore, some women are still interested in having ovarian tissue stored even though their own risk of POI is fairly low, as they simply do not want to risk being unable to have children. Other women wish to have their ovary cryopreserved, even when their estimated chances of survival are vague, because the chance of survival is present. When the woman herself needs to cover the cost of cryopreservation, for example as this service is offered in USA, the situation becomes even more difficult. It is therefore of utmost importance to give these women information about their specific situation: their estimated chances of surviving the cancer and furthermore an estimation of their fertility potential, and to inform them that the current experience is rather limited and it is not known to what extent women of, for instance, advanced reproductive age may actually benefit from having tissue transplanted. A parallel situation is seen in IVF treatment, where women of advanced reproductive age receive treatment despite the fact that the success rate is rather low.
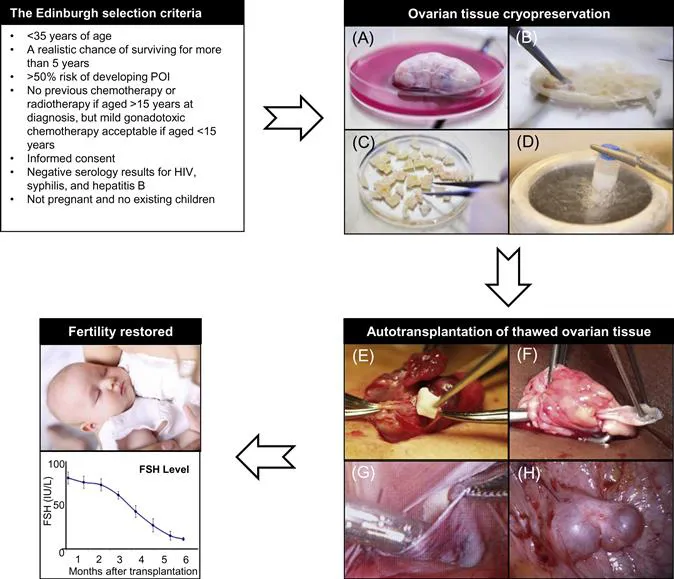
The Edinburgh selection criteria6 can be used as a guideline for fertility counselling. Cryopreservation of ovarian tissue: (A) Oophorectomy; one ovary or part of an ovary is surgically removed. (B,C) Tissue processing; the medulla is removed and the cortex is trimmed to a thickness of 1–2 mm and cut into pieces of 5×5 mm. The pieces of cortex equilibrate in cold freezing solution for 30 minutes on ice. (D) Freezing; the cortex pieces are transferred to individual cryotubes and slow-frozen in liquid nitrogen. Autotransplantation of thawed tissue: (E) Orthotopic transplantation; thawed pieces of cortex are placed in subcortical pockets in the remaining ovary. (F) Cortical strips (5×15 mm) transplanted to the ovary. (G) Heterotopic transplantation; thawed pieces of cortex are placed in a subperitoneal pocket corresponding to the pelvic wall. (H) Two human antral follicles observed at a heterotopic graft site several years after transplantation. Restored fertility: Restoration of ovarian function, i.e., serum levels of follicle-stimulating hormone (FSH; in IU/L) in 12 Danish patients after autotransplantation of frozen-thawed ovarian tissue (mean±SEM). HIV, Human immunodeficiency virus; POI, premature ovarian insufficiency.
7.2 Ovarian Tissue Cryopreservation
The success of ovarian cryopreservation is based on the high cryopreservation tolerance of small primordial follicles compared to the larger growing follicles. The vast majority of primordial follicles are located in the outermost 1 or 2 mm of the ovarian cortex, which is relatively easy to isolate from the rest of the ovarian tissue (see Figure 7.1). The frozen ovarian cortex is stored in liquid nitrogen, allowing time for the patient to recover after cancer treatment is completed.
The most widely used protocol for ovarian cryopreservation is the slow-freezing method, and up until now all live births in humans, except for one birth from Japan,7 have been achieved after slow-freezing.8 The most used media compositions include either dimethyl sulfoxide (DMSO) or ethylene glycol, both in combination with sucrose.4 However, vitrification of ovarian tissue could be one way of improving outcomes after freezing and re-implantation, as new results from non-human primates have shown superior follicular viability and development of antral follicles.9–13 Despite these encouraging results, the slow-freezing method is still preferred over vitrification.
Currently there is no consensus as to how much of the ovarian cortex should be harvested for cryopreservation. It is recommended that oophorectomy should be performed on patients receiving high doses of alkylating agents, patients undergoing pelvic irradiation or total body irradiation, and younger prepubertal girls due to the small size of their ovaries.8,14,15 For other patients, 4–5 ovarian cortical biopsies are harvested in some countries,8,16 whereas a unilateral oophorectomy is carried out systematically in other countries.17,18 The advantages of taking a whole ovary include minimizing the possibility of post-operational complications, e.g., bleeding, and also increasing the possibility of either grafting a large pool of follicles, which potentially should provide fertility with a higher efficacy, or performing repeated transplantation in case the tissue of the first transplantation becomes exhausted. However, the decision of excising o...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- List of Contributors
- Foreword
- Introduction
- Section I: Clinical
- Section II: Laboratory Models
- Section III: Strategies to Protect the Ovary