1.1 Introduction
Powders are particulate solid state materials containing discreet particles of size ranging from nanometres to millimetres. One gram of powder of the average particle size of 20 micron will contain around 108 particles. The bulk powder properties are the combined effect of particle properties. Food products in solid or liquid states are converted to powder form for ease of use, processing and keeping quality. Currently, many food products in the market are found in a powder form. The powder industries are growing tremendously around the globe aligned with the growth of industries that manufacture new products and powder premixes.
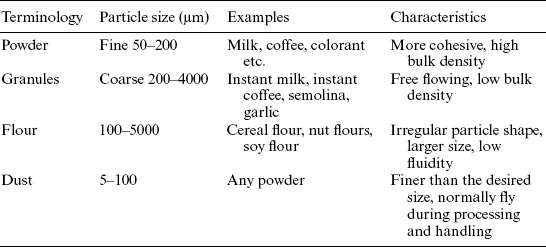
Various terms are used to indicate the particulate solids in bulk, such as powder, granules, flour and dust, though all these materials can be treated under powder category (Fig. 1.1, Table 1.1). These common terminologies are based on the size or the source of the materials. Granular products have a dimension on the order of millimetres while fine powder products are of average size less than 100 μm.
Table 1.1
Various terminologies used within the category of food powders
Fig. 1.1 Some examples of granules, powders and flour
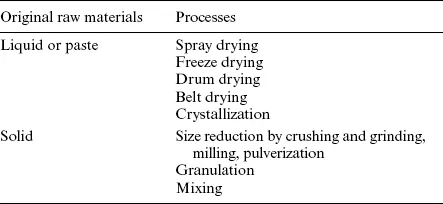
All types of powders are generally free flowing, can be used in very small portions (in milligrams) and are easy to transport in pipes pneumatically. In many processing situations, the powder forms are essential, such as in mixing and dissolution. Particles are created from bulk liquid or solid materials by drying, grinding, crushing, attrition, pulverization, precipitation or crystallization (Table 1.2). The two main methods of conversion of liquid to powder form are drying and crystallization. Size-reduction processes such as grinding and milling also contribute to powder production. The particle size, distribution, shape and surface characteristics and the density of the powders are highly variable and depend on both the characteristics of the raw materials and processing conditions during their formations. These parameters contribute to the functional properties of powders, including flowability, packaging density, ease of handling, dust forming, mixing/segregation, compressibility and surface activity. Powders have a large surface area per unit volume and may be hygroscopic (e.g., high degree of moisture absorption). The stability of a powder, in terms of physical and chemical properties, is usually impaired by increased moisture sorption (Bhandari and Hartel, 2005).
Table 1.2
Different methods to produce and process powders
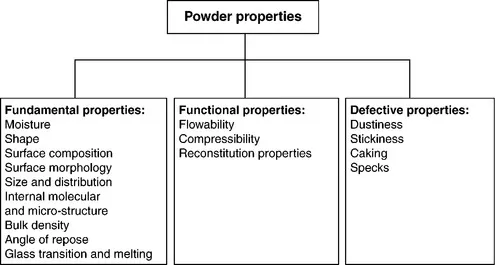
The properties of powder can be classified into three properties: fundamental, functional and defective (Fig. 1.2, Masters, 2002). The functional properties relate to the properties in relation to their application as a product or product ingredients and are directly influenced by the combination of fundamental properties. The defective properties are not desirable properties of the powders and often restrict their use for particular application.
Fig. 1.2 Three classes of powder properties.
1.2 Crystalline and amorphous microstructure of powders
Food powders may be amorphous, crystalline or mixed (semi-crystalline) in their molecular level structure. Depending on the process applied, the powders can be produced in either of these forms. Powders in crystalline state possess defined molecular alignment in the long-range order; while amorphous state is disordered. The properties of food powders including their functionality and their stability are highly dependent on these structures. Many of the desired and important properties of the food materials can be achieved by altering these structures.
1.2.1 Crystalline structure
Common powders found in crystalline states are salts, sugars and organic acids. Crystalline powders are non-hygroscopic, stable and easy flowing. The crystalline form is characterized by a tightly packed molecular arrangement ; therefore, only the molecules at the air-crystals interface can interact with external materials such as water (absorption). Thermodynamically, the crystalline form is in the lowest energy level or stable equilibrium state (Hartel, 2001). Normally, the crystallization process involves concentration of the solute above super-saturation by removal of the solvent through evaporation and/or by cooling. The crystals’ shape and size changes with the species, type of the polymorphs and isomers of same species. All these properties influence the final powder fundamental and functional properties. The crystalline powders are slower to dissolve than the amorphous powders as more energy and time are needed to dissociate the strongly bound molecules in a crystalline structure.
1.2.2 Amorphous structure
Amorphous structure exists in many important food powders such as high or low molecular weight carbohydrates and proteins. Many food products such as dairy powders, fruit juice powders, honey powders and hydrolyzed protein powders are normally in amorphous state. Molecules in the amorphous state are disordered, more open and porous. Therefore, an individual molecule possesses more sites for external interactions that make them able to absorb volatiles, for example, an amorphous structure can absorb water easily. The microstructure of an amorphous solid may consist of short-range order and regions of high and low densities and have higher entropy than the corresponding crystals. As the amorphous state is a non-equilibrium state, its structure can undergo crystallization or structural relaxation to achieve an equilibrium condition (Yu, 2001). Amorphous powders are obtained by rapid supercooling or rapid removal of solvent. Very short processing condition does not provide enough time for the molecules to align themselves to become crystalline. Spray drying is one of the most common methods used to produce amorphous powders. A material with slow crystallizing tendency promotes amorphous structure formation. Addition of impurities, mainly molecules of similar molecular structure (such as fructose in glucose) or large molecules (such as maltodextrins in sucrose), can prevent or delay crystallization and promote amorphous structure formation during drying. Addition of additives to control crystallization can create a completely amorphous or mixed (crystalline + amorphous) structure.
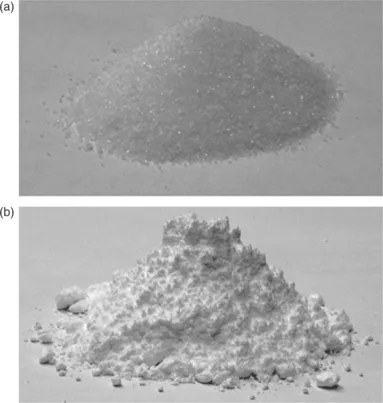
Cereal starches have semi-crystalline structure. Grinding cereals while making flour can destroy some of their crystalline structure, still maintaining its semi-crystalline structure. During the cooking process this crystalline structure is destroyed. This event is called gelatinization. Processes of melting the crystalline solid (such as sugar and starch) and rapid cooling post-grinding can also yield an amorphous powder. Although it is an energy intensive process, partial or completely amorphous structure can be formed from crystalline solids by milling or grinding them. A micronization process can virtually convert nearly all the crystalline arrangement to a non-equilibrium amorphous structure, though some microcrystallinity may always be present. One example is grinding of sugar crystals to make icing sugar (Fig. 1.3) which is a process of conversion of crystalline structure to semi-crystalline structure (further discussed in Section 1.2.3 ).
Fig. 1.3 Crystalline structure of sugar (a) and mixed structure (amorphous + crys...