1.1 Introduction
Funding is one of the critical inputs in the research and development process of biopharmaceuticals.1 For most projects and firms, funding represents a complex and dynamic process involving both corporate and government sources. Most firms directly and indirectly receive funding from a myriad of sources. These sources include other biopharmaceutical firms, venture capital investors, and state, federal, and regional governments.
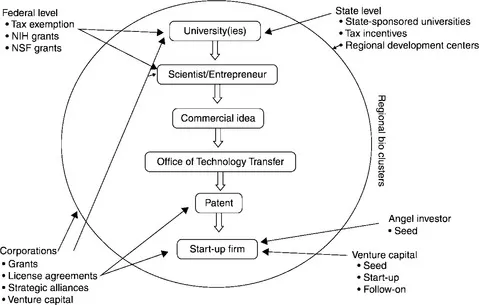
Figure 1.1 depicts a synopsis of these key stakeholders in the funding process for a university-based start-up company in the U.S. These individual components will be addressed in detail in later chapters. As can be seen in Figure 1.1, the federal government encourages research and development in this sector through the offering of grants to universities and researchers. The U.S. Bayh-Dole Act allows universities to retain ownership to inventions that receive federally funded research, thus creating a greater impetus for research to reach the market. Regional clusters are developing in areas where there are research-intense universities, existing corporations, and venture capital firms. States are supporting this development with tax incentives and regional development centers, hoping that their efforts will eventually lead to job creation within the cluster.2 Venture capital firms oftentimes help not just with the funding of these firms, but also in their creation as corporate entities. All of these funding sources work to foster the creation and development of biopharmaceutical products and processes. Interestingly, many countries and regions are adopting variations of this model to meet the needs of their own biopharmaceutical aspirations and market demands.
Figure 1.1 Key biopharmaceutical funding stakeholders
As a dynamic field, the biopharmaceutical market continues to rapidly expand and change. However, there remains no concise agreed upon definition in the literature as to what precisely is a biopharmaceutical firm,3 as both the chemical-based pharmaceutical industry and the biological-based biotechnology industry have a shared and intertwined history.4 This book takes a practitioner’s view and divides the biopharmaceutical market sector into biotechnology firms (and industry), pharmaceutical firms (and industry), and biopharmaceutical firms (i.e., firms that are involved with both biotechnology and pharmaceutical products and processes). In addition, various biopharmaceutical firms may serve a range of biopharmaceutical purposes. These purposes or types can be described as human, agricultural (both plant and animal), industrial, and military. This book focuses on human biopharmaceuticals. It notes that just as it is oftentimes difficult to disaggregate the data related to pharmaceutical firms from biotechnology firms, so too is it difficult at times to separate the work of firms focusing on human drugs or other products from firms focusing on agricultural, industrial, or military drugs or other products. This is because often a firm’s work has multiple purposes or uses or the firm seeks to diversify within this market sector. Also biopharmaceutical firms are sometimes labeled life sciences firms which include medical device firms and others. This book will at times separate the biopharmaceutical firms’ data from other firms’ data when possible and note otherwise.
1.2 Synopsis of the development of the biopharmaceutical market sector
The modern pharmaceutical industry began in the mid-nineteenth century with the development of the synthetic dye industry in Germany and Switzerland. German and Swiss chemical firms such as Ciba, Bayer, and Sandoz were discovering and manufacturing dyestuffs and other organic chemicals, which showed medicinal effects.4,5 Prior to World War I, German companies produced roughly 80% of the world’s pharmaceutical products.5 The U.S. during this time was industrializing at a rapid pace. However, it lagged behind German science-based industries such as the pharmaceutical industry, in part due to the U.S.’s lack of research-oriented universities and medical schools.6
Emerging pharmaceutical firms in the U.S. and the U.K. at this time were much smaller and primarily focused on the development of new drugs, whereas their German and Swiss counterparts remained diversified chemical companies with drug development being one aspect of their overall operations. After the Flexner Report on medical education in 1910, U.S. medical schools came to imitate their German counterparts to some degree and developed a more scientific approach to the understanding and practice of medicine based on anatomy and physiology. This Western understanding and practice of medicine4 based in the university and utilizing the scientific method had a profound effect on Western medicine and the biopharmaceutical market sector in particular.
A history of the West’s scientific advancements in this area is beyond the scope of this book. However, several foundational early discoveries and developments that furthered the Western tradition of medicine and biopharmaceuticals are worthy of mention. These include: the invention of the multi-lens or compound microscope by Zaccharias and Hans Janssen in 1590 which led to the eventual discovery of bacteria; the publication in 1859 of The Origin of Species by the English naturalist Charles Darwin; the Austrian abbot Gregor Mendel’s discovery of the laws of genetic inheritance in the 1860s; Louis Pasteur’s work on the role of living microorganisms in fermentation which helped found microbiology as a scientific discipline; Friedrich Miescher’s isolation of DNA in 1869; Robert Koch’s discovery that bacteria causes disease which led to the foundation of medical bacteriology in 1870; and the development of biochemistry by Emil Fischer and Eduard Buchner in the 1880s and 1890s.3–5
These advancements have far-reaching implications not just for the West, but also elsewhere. By the mid-1800s, Japan begins to adopt the German system of medical education, with Taiwan and Korea also proceeding to adopt this method shortly thereafter.4 China, on the other hand, even today continues to have a mixed approach with both Western based and traditional Chinese methods of treatment.
In 1928, the Scottish bacteriologist Alexander Fleming discovered penicillin.4 The need for large quantities of penicillin during World War II led to several significant innovations in the U.S. First, the U.S. government became involved on an unprecedented level seeking to fund and coordinate efforts among companies, universities, and governmental departments.3 This is not unlike the very early days of Silicon Valley (see Lerner, 2009).7 Second, companies invested heavily in internal research and development units to support this effort. Prior to this, few U.S. firms did major research. Third, the general public in the U.S. began to recognize and support health related research.5 These innovations helped the further development of companies such as Eli Lilly, Merck, Pfizer, and Squibb.
After World War II, these pharmaceutical firms and others reaped the benefits of their newly expanded research and development (R&D) departments and funding. Prior to the war and the development of penicillin, there were few developed drugs that cured diseases, as most merely treated symptoms.5 After the war, through the development of these pharmaceutical firms’ R&D efforts, there was an explosion of highly profitable drugs offered on the market, leading to a period known as the golden age of pharmaceuticals.5 This was a period where the drug development process known as random screening took place. In this approach, firms randomly screened compounds in test tube and laboratory animal experiments for potential therapeutic activity. Literally thousands of compounds were screened for potential substances, with the results kept (but rarely shared) in the firm’s libraries. Oftentimes, firms seeking a cure for one disease happened upon a treatment for another disease. Chance was a common theme in this process.5
Although the process worked well for a time, it did not lead to a c...