
- 432 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Nuclear and Radiochemistry
About this book
The field of nuclear and radiochemistry is wide-reaching, with results having functions and use across a variety of disciplines. Drawing on 40 years of experience in teaching and research, this concise book explains the basic principles and applications of the primary areas of nuclear and radiochemistry. Separate chapters cover each main area of recent radiochemistry. This includes nuclear medicine and chemical aspects of nuclear power plants, namely the problems of nuclear wastes and nuclear analysis (both bulk and surface analysis), with the analytical methods based on the interactions of radiation with matter. Furthermore, special attention is paid to thermodynamics of radioisotope tracer methods, the very diluted system (carrier-free radioactive isotopes) and the principles of chemical processes with unsealed radioactive sources. This book will be helpful to students and researchers in chemistry, chemical engineering, environmental sciences, and specialists working in all fields of radiochemistry.
- Basic concepts are introduced and practical applications explained, providing a full view of the subject.
- Laboratory work with unsealed radiochemicals is discussed in details that can be applied in research and authority in the lab environment.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction
From the dawn of natural sciences, scientists and philosophers have reflected on the nature of matter. In the end of the nineteenth century, the discoveries signed by Lavoisier, Dalton, and Avogadro (namely, the law of conservation of mass, the atomic theory, and the definition of a mole as a unit of the chemical quantity) led to a plausible model. This model was built on the principles of Dalton’s atomic theory, which states that:
• all matter is composed of small particles called atoms,
• each element is composed of only one chemically distinct type of atom,
• that all atoms of an element are identical, with the same mass, size, and chemical behavior, and
• that atoms are tiny, indivisible, and indestructible particles.
In the same period, the basic laws of thermodynamics have been postulated. The first law of thermodynamics is an expression of the principle of conservation of energy.
This model of the matter has been challenged when it was discovered that the same element can have radioactive and stable forms (i.e., an element can have atoms of different mass). The discovery of the radioactivity is linked to Henri Becquerel’s name and to the outcome of his experiments which were presented in 1896 at the conference of the French Academy and published in Comptes Rendus e l’Académie des Sciences.
Following his family tradition (his father and grandfather also studied fluorescence, and his father, Edmund Becquerel, studied the fluorescence of uranium salts), Becquerel examined the fluorescent properties of potassium uranyl sulfate [K2UO2(SO4)2·2H2O]. Since Wilhelm Röntgen’s previous studies, it has been known that X-rays can be followed by phosphorescent light emitted by the wall of the X-ray tube, and Becquerel wanted to see if this process could be reversed, i.e., if phosphorescent light can produce X-rays. After exposing potassium uranyl sulfate to sunlight, he wrapped it in black paper, placed it on a photographic plate, and observed the “X-ray.” He repeated the experiments with and without exposure to sunlight and obtained the same result: the blackening of the photographic plate. He has concluded that the blackening of the photographic plate was not caused by fluorescence induced by sunlight, but rather by an intrinsic property of the uranium salt. This property was first called Becquerel rays, and later it was termed “radioactive radiation1.” Becquerel also has observed that electroscope loses its charge under the effect of this radiation because the radiation induces charges in the air.
The same radiation was observed by Pierre Curie and Marie Curie, as well as G. Schmidt in Germany using thorium salts. They have found that the ores of uranium and thorium have more intense radiation than the pure salts: for example, pitchblende from Johanngeorgenstadt and Joachimstal has about five and four times more intense radiation, respectively, than black uranium oxide (U3O8). This more intense radiation originates from elements that were not present in the pure salts, which later were identified as the new radioactive elements polonium and radium, and which were separated from uranium ore in Joachimstal. The Curies presented the results at the French Academy in 1898 and published in Comptes Rendus e l’Académie des Sciences. As proposed by Marie Curie, the first new radioactive element, polonium, was named after her homeland of Poland. In the Curies’ laboratory, radioactivity was detected by the ionization current produced by the radiation. In 1902, the Curies produced 100 mg of radium and determined the atomic mass, which they later corrected (226.5 g/mol). Marie Curie produced metallic radium by electrolysis of molten salts in 1910.
Rutherford has differentiated three types of radiation (alpha, beta, and gamma) by using absorption experiments in 1889. He also determined that the radiations had very high energy. In 1903, Rutherford and Soddy concluded that the radioactive elements are undergoing spontaneous transformation from one chemical atom into another and that the radioactive radiation was an accompaniment of these transitions. Radioactive elements were called radioelements. Since they were not known earlier, and therefore did not have names, some of them were named by adding letters to the name of the original (i.e., parent) element (e.g., UX, ThX). Others were given new names (such as radium, polonium, radium emanation-today radon).
The discovery of radium and polonium filled two empty places on the periodic table. Later studies, however, showed that some radioactive elements had the same chemical properties as known stable elements—they differed only in the amount of radioactivity. Therefore, they should be put in places in the periodic table that are already filled, which is impossible according to Dalton’s atomic theory. For example, different types of thorium (thorium, UX1, iononium (Io), radioactinium, today Th-232, Th-234, Th-230, and Th-227, respectively) and radium (radium, mesothorium1, ThX, AcX, today Ra-226, Ra-228, Ra-224, or Ra-223, respectively) atoms have been recognized.
These experimental results presented serious contractions to the Daltonian model of matter and the principle of the conservation of mass and energy. Einstein has solved part of these contradictions using the law of the equivalence of energy and mass:
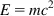
where E is the energy of the system, m is the mass, and c means the velocity of light in a vacuum.
As the interpretation of the other part of the contradictions, Soddy defined the term “isotopes,” neglecting the postulate in Dalton’s theory on the identity of the atoms of an element. Accordingly, isotopes are atoms of the same element having different masses.
What kind of scientific and practical importance did these discoveries have? At first, they formed the basis of the modern atomic theory, resulting in the development of new fields and explaining some phenomena. For example, nucleogenesis, the formation of the elements in the universe, now can be explained based on the principles of natural sciences, attempting to give a philosophical significance of the “creation.”
From the beginning, the practical importance has been underestimated. In 1898, however, radium found its role in cancer therapy. In 1933 in the Royal Society meeting, Rutherford said that “any talk of atomic energy” was “moonshine.” Rutherford’s statement inspired Leo Szilárd to devise the principle of the nuclear...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- 1. Introduction
- 2. Basic Concepts
- 3. Isotopes
- 4. Radioactive Decay
- 5. Interaction of Radiation with Matter
- 6. Nuclear Reactions
- 7. Nuclear Energy Production
- 8. Radioactive Tracer Methods
- 9. Physicochemical Application of Radiotracer Methods
- 10. Radio- and Nuclear Analysis
- 11. Industrial Application of Radioisotopes
- 12. An Introduction to Nuclear Medicine
- 13. Environmental Radioactivity
- 14. Detection and Measurement of Radioactivity
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Nuclear and Radiochemistry by Jozsef Konya,Noemi M. Nagy in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.