
- 432 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About This Book
In recent years, there have been considerable developments in techniques for the investigation and utilisation of enzymes. With the assistance of a co-author, this popular student textbook has been updated to include techniques such as membrane chromatography, aqueous phase partitioning, engineering recombinant proteins for purification and due to the rapid advances in bioinformatics/proteomics, a discussion of the analysis of complex protein mixtures by 2D-electrophoresis and RPHPLC prior to sequencing by mass spectroscopy. Written with the student firmly in mind, no previous knowledge of biochemistry, and little of chemistry, is assumed. It is intended to provide an introduction to enzymology, and a balanced account of all the various theoretical and applied aspects of the subject which are likely to be included in a course.
- Provides an introduction to enzymology and a balanced account of the theoretical and applied aspects of the subject
- Discusses techniques such as membrane chromatography, aqueous phase partitioning and engineering recombinant proteins for purification
- Includes a discussion of the analysis of complex protein mixtures by 2D-electrophoresis and RPHPLC prior to sequencing by mass spectroscopy
Frequently asked questions
Information
An Introduction to Enzymes
1.1 WHAT ARE ENZYMES?
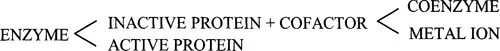
1.2 A BRIEF HISTORY OF ENZYMES
1.3 THE NAMING AND CLASSIFICATION OF ENZYMES
1.3.1 Why classify enzymes?
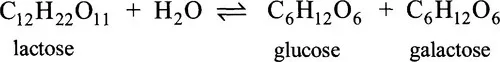
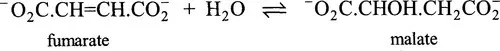
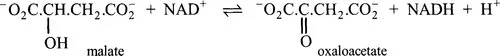
1.3.2 The Enzyme Commission’s system of classification
| First digit | Enzyme class | Type of reaction catalysed |
| 1 | Oxidoreductases | Oxidation/Reduction reactions |
| 2 | Transferases | Transfer of an atom or group between two molecules (excluding reactions in other classes) |
| 3 | Hydrolases | Hydrolysis reactions |
| 4 | Lyases | Removal of a group from substrate (not by hydrolysis) |
| 5 | Isomerases | Isomerization reactions |
| 6 | Ligases | The synthetic joining of two molecules, coupled with the breakdown of the pyrophosphate bond in a nucleoside triphosphate |
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Authors’ Preface
- Part 1: Structure and Function of Enzymes
- Part 2: Kinetic and Chemical Mechanisms of Enzyme-Catalysed Reactions
- Part 3: Application of Enzymology
- Answers to Problems
- Abbreviations
- Index