
- 256 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Materials for Engineering
About this book
This third edition of what has become a modern classic presents a lively overview of Materials Science which is ideal for students of Structural Engineering. It contains chapters on the structure of engineering materials, the determination of mechanical properties, metals and alloys, glasses and ceramics, organic polymeric materials and composite materials. It contains a section with thought-provoking questions as well as a series of useful appendices. Tabulated data in the body of the text, and the appendices, have been selected to increase the value of Materials for engineering as a permanent source of reference to readers throughout their professional lives. The second edition was awarded Choice's Outstanding Academic Title award in 2003. This third edition includes new information on emerging topics and updated reading lists.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part I
Characterization of engineering materials
1
Structure of engineering materials
1.1 Crystal structure
Crystal structure refers to the ordering of atoms into different crystalline arrangements. It is the arrangement of these atoms – the strength and directionality of the interatomic bonds – which determines the ultimate strength of the solid. Techniques involving X-ray or electron diffraction are employed to determine crystal structures, and four types of interatomic bonding are recognized: van der Waals, covalent, ionic and metallic. The latter three ‘primary’ bonds are limiting cases, however, and a whole range of intermediate bonding situations also exist in solids.
The van der Waals force is a weak ‘secondary’ bond and it arises as a result of fluctuating charges in an atom. There will be additional forces if atoms or molecules have permanent dipoles as a result of the arrangement of charge inside them. In spite of their low strength, these forces can still be important in some solids; for example it is an important factor in determining the structure of many polymeric solids.
Many common polymers consist of long molecular carbon chains with strong bonds joining the atoms in the chain, but with the relatively weak van der Waals bonds joining the chains to each other. Polymers with this structure are thermoplastic, i.e. they soften with increasing temperatures and are readily deformed, but on cooling they assume their original low-temperature properties and retain the shape into which they were formed.
Covalent bonding is most simply exemplified by the molecules of the non-metallic elements hydrogen, carbon, nitrogen, oxygen and fluorine. The essential feature of a covalent bond is the sharing of electrons between atoms, enabling them to attain the stable configuration corresponding to a filled outermost electron shell. Thus, an atom with n electrons in that shell can bond with only 8 – n neighbours by sharing electrons with them.
For example, when n = 4, as in carbon in the form of diamond, one of the hardest materials known, each atom is bonded equally to four neighbours at the corners of a regular tetrahedron and the crystal consists of a covalent molecule, Fig. 1.1(a). In graphite, only three of the four electrons form covalent bonds, so a layer structure forms, Fig. 1.1(b), and the fourth electron is free, which gives some metallic properties to this form of carbon. Graphite crystals are flat and plate-like, and they are so soft that graphite is used as a lubricant. It is clear from Fig. 1.1 that the different dispositions of the covalent bonds in space have a profound influence on the atomic arrangements and hence upon properties of the material.
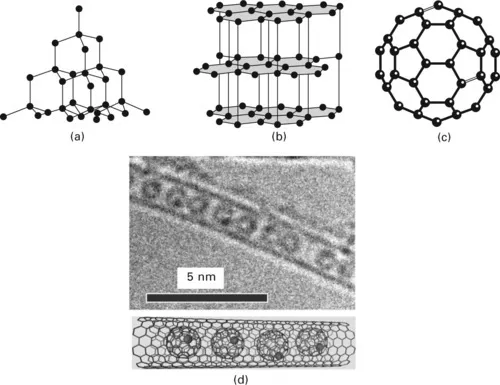
1.1 Crystal structure of (a) diamond; (b) graphite; (c) buckminsterfullerene, C60; and (d) electron micrograph of a series of buckeyballs within a carbon nanotube: a diagram of the structure is shown underneath. (Courtesy Dr Andrei Khlobystov.)
For many years diamond and graphite were the only known forms of carbon, but, in 1985, a new form of carbon (buckminsterfullerene), C60, was identified, Fig. 1.1(c), as a soccer-ball-like cage of 60 carbon atoms with a diameter of 0.71 nm. This was the only allotrope of any element to have been discovered in the twentieth century. Other, larger, fullerene ‘buckeyballs’ have subsequently been discovered and, in 1991, multiwalled carbon nanotubes were discovered. Two years later, single-walled carbon nanotubes were discovered with diameters generally varying between 1.3 and 1.6 nm. Figure 1.1(d) is an electron micrograph showing a series of fullerene buckeyballs within a carbon nanotube. Carbon nanotubes can be synthesized by a number of techniques, including carbon arcs, laser vaporization and ion bombardment. They consist of concentric, cylindrical, graphitic carbon layers capped on the ends with fullerene-like domes.
The possibility of encapsulating atoms (and...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Preface to the third edition
- Preface to the second edition
- Preface to the first edition
- Introduction
- Part I: Characterization of engineering materials
- Part II: Structure–property relationships
- Part III: Problems
- Part IV: Appendices
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Materials for Engineering by J Martin in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Materials Science. We have over one million books available in our catalogue for you to explore.