![]()
Chapter 1
Affinity Chromatography
D.S. Hage, J.A. Anguizola, R. Li, R. Matsuda, E. Papastavros, E. Pfaunmiller, M. Sobansky and X. Zheng, Chemistry Department, University of Nebraska, Lincoln
Outline
1.1. Introduction
1.2. Basic Components of Affinity Chromatography
1.3. Bioaffinity Chromatography
1.4. Immunoaffinity Chromatography
1.5. Dye-Ligand and Biomimetic Affinity Chromatography
1.6. Immobilized Metal-Ion Affinity Chromatography
1.7. Analytical Affinity Chromatography
1.8. Miscellaneous Methods and Newer Developments
Acknowledgement
References
1.1 Introduction
Affinity chromatography is a type of liquid chromatography in which a biologically related agent is used in a column as a stationary phase to purify or analyze the components of a sample [1–4]. The ability of this method to selectively bind and purify its target compounds is based on the specific and reversible interactions present in many biological systems, such as the binding of a hormone to a receptor or an antibody to its antigen.
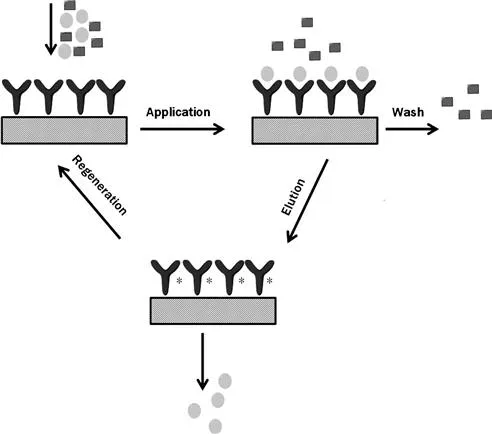
To develop a method based on affinity chromatography, one of the pair of interacting species is first immobilized to a solid support, such as agarose beads or silica particles. The immobilized agent, called the affinity ligand, acts as the stationary phase for the affinity column [1,3]. The other interacting compound is then injected onto the affinity column or applied in the presence of an application buffer, which allows the desired target to bind to the immobilized ligand (see Figure 1.1) [5]. After nonretained sample components have been washed from the column, the retained target analyte is typically released in the presence of an elution buffer [2,3,5]. If the retained compound has only weak or moderate binding to the immobilized ligand, it is also possible to use the application buffer to elute this target under isocratic conditions; this approach is known as weak-affinity chromatography (WAC) [5–11]. As the target elutes, it is collected for further use or analyzed by an on-line or off-line detector. The column is regenerated by reapplying the application buffer before the next sample injection.
FIGURE 1.1 Typical scheme for the application of a sample to an affinity column, elution of the retained targets, and regeneration of the affinity column. [1]
1.2 Basic Components of Affinity Chromatography
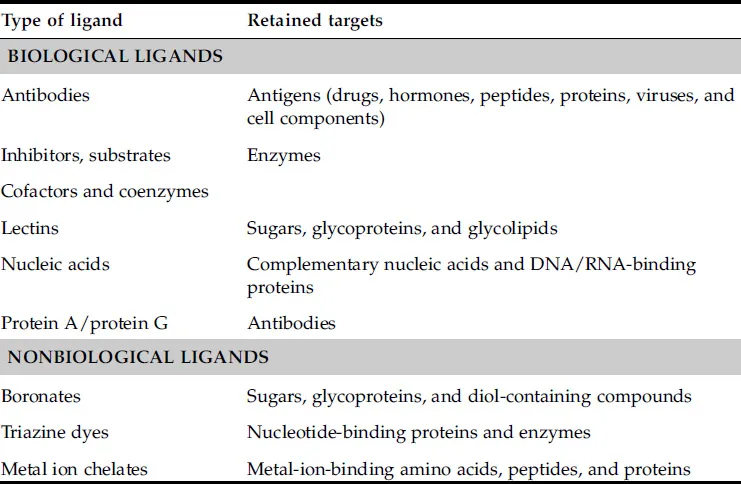
The success of any affinity separation depends largely on the selection of the ligand immobilized in the column. A summary of common ligands used in affinity chromatography is given in Table 1.1. Many of the ligands used in affinity chromatography are obtained from a biological source, such as antibodies, serum proteins, and lectins. Other binding agents that are useful in affinity chromatography are boronic acid, metal chelates, and triazine dyes, which are synthetic agents or inorganic molecules that can be employed as ligands [1–4].
TABLE 1.1
Common Ligands Used in Affinity Chromatography
Source: This table is based on information provided in Refs. [1–4].
The affinity ligands can be divided into two main categories: high-specificity ligands and general ligands [3]. High-specificity ligands are binding agents that retain only one or a few closely related targets; these ligands are used when the goal is to isolate or separate a specific solute. Examples of high-specificity ligands include antibodies for the binding of antigens, substrates or inhibitors for separating or binding enzymes, and single-strand nucleic acids for the retention of complementary sequences of DNA or RNA [1]. General ligands are binding agents that retain a class of related molecules or structurally similar targets. Examples of general ligands are lectins and boronates for binding carbohydrate-containing agents, some types of dyes for the retention of enzymes and proteins, and protein A or protein G for the binding of immunoglobulins [3].
When designing a system for use with affinity chromatography, an important aspect to consider is the selection of the support material utilized for ligand attachment. This support material should have low nonspecific binding for sample components but be easy to modify for chemical activation and ligand attachment. In addition, the support should be able to withstand the pressures and flow rates used in the separation [1]. Agarose is often used as a support in traditional affinity columns [1]. This material consists of polymeric chains comprised of D-galactose and 3,6-anhydro-L-galactose [12]. Another common type of polysaccharide support is cellulose. Work has also been conducted in the use of affinity ligands with supports for high-performance liquid chromatography (HPLC), resulting in a method known as high-performance affinity chromatography (HPAC) or high-performance liquid affinity chromatography (HPLAC) [1]. Supports utilized in this latter method include silica particles, modified polystyrene supports, silica monoliths, and organic-based monoliths [12–15].
Several techniques are available for attaching a ligand to a chromatographic support. These techniques include both covalent and nonspecific immobilization methods [16]. Nonspecific immobilization techniques involve the physical adsorption of a ligand to a support [17,18]. Biospecific adsorption is a form of noncovalent immobilization that uses the binding between two ligands, one of which has been previously bound to the support and the second of which is adsorbed to the first ligand and used to bind the final desired target. An example of this approach is in the use of avidin or streptavidin for the adsorption of biotinylated proteins [16,19]. Another example is the use of immobilized protein A or protein G to adsorb antibodies for use in the creation of immunoaffinity supports [16,20,21]. Covalent immobilization involves the chemical attachment of a ligand to a chromatographic support. In this method, the support must first be activated for ligand attachment. Several functional groups can be used for covalent immobilization. These methods may involve amine groups, carboxyl groups, sulfhydryl groups, hydroxyl groups, or aldehyde groups [16].
The application buffer used with an affinity column should have an appropriate pH and ionic strength to promote binding between the immobilized ligand and target [5]. The elution buffer is a mobile phase that disrupts the binding of the target with the ligand. This elution may be accomplished by changing the pH, ionic strength, or amount of organic solvent in the mobile phase. Such an approach, referred to as nonspecific elution, is often used in analytical methods for the quick removal of a target from an affinity column. Alternatively, a competing agent may be placed into the mobile phase to displace the target from the column by binding with either the target or ligand, thus preventing their further interaction. This method is called biospecific elution. Although biospecific elution is often slower than nonspecific elution, it is useful when gentle removal and affinity purification of an active target are desired [5].
1.3 Bioaffinity Chromatography
Bioaffinity chromatography is a type of affinity chromatography that employs a biological ligand as the stationary phase [22]. This technique was first used in 1910 by Starkenstein to purify α-amylase using insoluble starch. Bioaffinity chromatography is now commonly used as a purification technique for numerous compounds [23]. Because many biological agents are found in nature, bioaffinity chromatography is one of the most diverse forms of affinity chromatography. Several examples of biological agents that can be used as ligands in bioaffinity applications are listed in Table 1.1. Many of these agents are commercially available in an immobilized form for use in affinity columns [3,22,24].
Enzyme purification was the first application of bioaffinity chromatography [23] and remains an important use of this technique. In this type of separation, ligands, such as enzyme inhibitors, coenzymes, or cofactors, are used to purify and separate enzymes [25]. For instance, in 1968 and the first report of “modern” affinity chromatography, Cuatrecasas, Wilchek, and Anfinsen employed specific enzyme inhibitors to selectively isolate enzymes [1,4]. A more recent example was the use of a support containing flavin mononucleotides for the purification of flavin adenine dinucleotide synthetase [26]. Other examples have included the use of mono-, di-, and triphosphate nucleotides for the purification of kinases and the use of nicotinamide adenine dinucleotide for the isolation of dehydrogenases [26,27].
Lectins are another group of ligands that are frequently found in bioaffinity chromatography. Lectins are nonimmune system proteins able to bind to specific carbohydrate groups [22]. The most common lectins used in bioaffinity chromatography are concanavalin A (Con A), wheat-germ agglutinin (WGA), and jacalin [25]. Con A has the ability to bind to targets that contain α-D-mannose or α-D-glucose residues [25]. WGA specifically binds to D-N-acetylglucosamine residues, and jacalin binds to galactose or mannose residues [25,28]. The most popular application for lectins has been in glycomics, where these ligands are used to isolate and separate polysaccharides, glycoproteins, glycopeptides, and glycolipids [24,25]. For instance, supports containing multiple lectins (e.g., Con A, WGA, and jacalin) have been used for the separation of glycoproteins in plasma samples to help map glycosylation patterns for disease detection [29,30].
Immunoglobulin-binding proteins are another class of ligands that are employed in bioaffinity chromatography. Protein A and protein G are two examples of such binding agents. Protein A is produced by Staphylococcus aureus and protein G is produced by group G streptococci [22,31–33]. Both proteins bind to the Fc region of immunoglobulins [22,25], which makes these ligands useful for antibody purification and the biospecific adsorption of antibodies to affinity supports [25]. Protein A and protein G have some differences in the species and classes of antibodies to which they will bind [22,31–33]. For example, protein A can bind to human antibodies that belong to the classe...