
- 538 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Efficient Methods for Preparing Silicon Compounds
About this book
Efficient Methods for Preparing Silicon Compounds is a unique and valuable handbook for chemists and students involved in advanced studies of preparative chemistry in academia and industry. Organized by the various coordination numbers (from two to six) of the central silicon atom of the reported compounds, this book provides researchers with a handy and immediate reference for any compound or properties needed in the area.Edited by a renowned expert in the field, each chapter explores a different type of compound, thoroughly illustrated with useful schemes and supplemented by additional references. Knowledgeable contributors report on a broad range of compounds on which they have published and which are already used on a broad scale or have the potential to be used in the very near future to develop a new field of research or application in silicon chemistry.- Includes contributions and edits from leading experts in the field- Includes detailed chemical schemes and useful references for each preparative method- Organized by the coordination numbers of the central silicon atom for each compound for easy navigation- Serves as a go-to primer for researchers in novel compositions of silicon matter
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Arylsilanes as Precursors of Cyclohexa-2,5-dienylsilanes
Abstract
Keywords
Arylsilanes; Birch reduction; Cyclohexadienes; Electrochemistry; HMPA; Sacrificial anode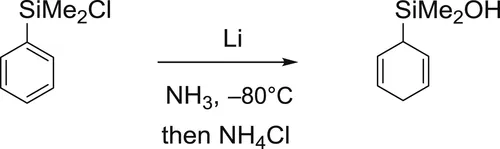
Preparation of cyclohexa-2,5-dienyldimethylsilanol
Apparatus
Chemicals
Experimental procedure
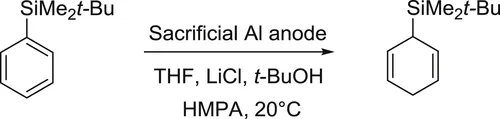
Apparatus
Chemicals
Attention!
Experimental procedure
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- List of Contributors
- Preface
- 1. Arylsilanes as Precursors of Cyclohexa-2,5-dienylsilanes
- 2. Rhodium-Catalyzed Vinyldiazoesters Insertion Into SiH Bonds. Synthesis of Allylsilanes
- 3. Lewis Base–Stabilized Silyliums
- 4. Tetra(silyl)methane, (H3Si)4C, a Volatile Carbosilane for the Chemical Vapor Deposition of Amorphous Silicon Carbide Thin Films
- 5. Trimethylsilyl Perrhenate—A Nonionic Reagent Soluble in Organic Solvents for the Preparation of Perrhenates
- 6. Radicals, Anions, and Cations of Silicon and Silylenes
- 7. Multiple Bonding in Silicon Compounds
- 8. Silicon-Based Ligands for Transition Metal Coordination
- 9. Silylenes, Silylaminosilylene, Disilane, Silanimine, Silacyclohexadienones, Bis(silyl)-Alkenes, and Hydrosilanimine
- 10. Synthesis of Functionalized Silsesquioxanes as Molecular Templates for Hybrid Materials
- 11. Lithium Tris(2,4,6-triisopropylphenyl)disilenide: A Versatile Reagent for the Transfer of the Disilenyl Group
- 12. New Phosphine-Stabilized Si(II)-Complexes: Silicon(II)-Hydride and Silacyclopropylidene
- 13. (Monosodiumoxy)organoalkoxysilanes (Rebrov Salts)—Polyfunctional Monomers for Silicone Syntheses
- 14. Silicon(II) as a Synthon for the Access of Different Silicon(IV) and Silicon(II) Compounds
- 15. Silene, Silaimine, and Siletane Derivatives
- 16. Synthesis of a Zwitterionic 2,4-Disila-1,3-diphosphacyclobutadiene Compound
- 17. Silanetriols and Aluminosilicates
- 18. Synthesis of Silicon(II) Compounds and Their Reactions
- 19. Preparation of the NHC (L1,2) and Its Application for Synthesizing Lewis Base–Stabilized Dichlorosilylene L1,2SiCl2
- 20. Octaammonium POSS as a Building Block for Constructing Nanohybrid Materials
- 21. Tungsten- and Ruthenium-Silylene Complexes
- 22. 1,1-Di-tert-Butylsilacyclopropanes
- 23. Polysilanes, Polycarbosilanes, Dioxadisilacyclohexane, and Polysiloxanes
- 24. Synthesis of N-(Silylmethyl)amides of Carboxylic Acids and Related Compounds
- 25. Carbene Adducts of Silicon(IV) Chlorides: Versatile Reagents for Carbene Transfer and Sources for Cationic Silicon(IV) Complexes
- 26. Controlling n-Oligosilane Conformation by Stretching on a Staffane Rack
- 27. Bis-silyl Chelate Ligand Precursor XantsilH2 and Some Ruthenium Xantsil Complexes
- 28. Silyl(silylene) Complexes of Iron and Ruthenium
- 29. Cobalt-Methylidyne-Silanetriol as Precursor for Catalytic Hydroformylation in a Two-Phase System
- 30. Preparation of the SiCS Three- and the SiO2C2 Five-Membered Ring System
- 31. Preparation of SiF4(NH3)2 and Its Higher Ammoniate SiF4(NH3)2·2NH3
- 32. Silanols and Silsesquioxanes
- 33. Hydrido-Silyl Complexes of Chromium With Metal-Hydrogen-Silicon Three-Center Bonds
- 34. Sol-Gel Processing of Alkoxysilyl-Substituted Metal Complexes
- 35. Tertiary Alkyl Substituted Octasilsesquioxanes
- 36. o-(Dimesitylboryl)(dimethylsilyl)benzene: A System of Intramolecular SiH Bond Activation by o-Boryl Group
- 37. Organosilicon Synthesis for Construction of Organosilicon Clusters
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app