
Fluorine and Health
Molecular Imaging, Biomedical Materials and Pharmaceuticals
- 816 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Fluorine and Health
Molecular Imaging, Biomedical Materials and Pharmaceuticals
About This Book
Fluorine and Health presents a critical multidisciplinary overview on the contribution of fluorinated compounds to resolve the important global issue of medicinal monitoring and health care. The involved subjects are organized in three thematic parts devoted to Molecular Imaging, Biomedical Materials and Pharmaceuticals. Initially the key-position of partially fluorinated low molecular weight compounds labelled either with the natural 19F-isotope for Magnetic Resonance Imaging (MRI) or labelled with the radioactive [18F]-isotope for Positron Emission Tomography (PET) is highlighted. Both non-invasive methods belong to the most challenging in vivo imaging techniques in oncology, neurology and in cardiology for the diagnosis of diseases having the highest mortality in the industrialized countries. The manifold facets of fluorinated biomaterials range from inorganic ceramics to perfluorinated organic molecules. Liquid perfluorocarbons are suitable for oxygen transport and as potential respiratory gas carriers, while fluorinated polymers are connected to the pathology of blood vessels. Another important issue concerns the application of highly fluorinated liquids in ophthalmology. Moreover, fluorine is an essential trace element in bone mineral, dentine and tooth enamel and is applied for the prophylaxis and treatment of dental caries. The various origins of human exposure to fluoride species is detailed to promote a better understanding of the effect of fluoride species on living organisms.Medicinally relevant fluorinated molecules and their interactions with native proteins are the main focus of the third part. New molecules fluorinated in strategic position are crucial for the development of pharmaceuticals with desired action and optimal pharmacological profile. Among the hundreds of marketed active drug components there are more than 150 fluorinated compounds. The chapters will illustrate how the presence of fluorine atoms alters properties of bioactive compounds at various biochemical steps, and possibly facilitate its emergence as pharmaceuticals. Finally the synthetic potential of a fluorinase, the first C-F bond forming enzyme, is summarized.
- New approach of topics involving chemistry, biology and medicinal techniques
- Transdisciplinar papers on fluoride products
- Importance of fluoride products in health
- Updated data on specific topics
Frequently asked questions
Information
Fluorine‐18 Chemistry for Molecular Imaging with Positron Emission Tomography
Abstract
1 INTRODUCTION
2 THE RADIONUCLIDE FLUORINE‐18 AND SOME GENERAL CONSIDERATIONS CONCERNING SHORT‐LIVED POSITRON EMITTERS
2.1 The position of fluorine‐18 among short‐lived positron emitters for PET
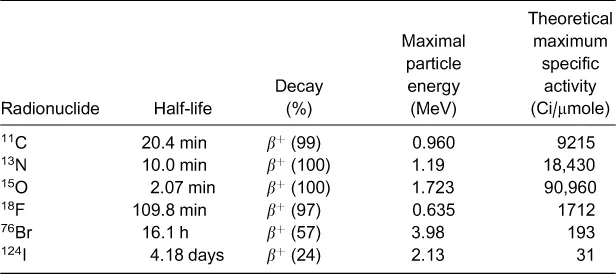
2.2 Design of radiotracers and radiopharmaceuticals labelled with a short‐lived positron emitter: The case of fluorine‐18
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributors
- Preface
- Part I: Molecular Imaging
- Part II: Biomedical Materials
- Part III: Pharmaceuticals
- Subject Index