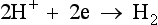General Technique
During an electrochemical reaction, electrons are transferred between a molecule of the substrate and the electrode. Electrons are always transferred singly and the substrate first is converted to an intermediate with an unpaired electron. Transformation of this reactive intermediate to the final product involves a sequence of bond forming or bond cleaving reactions and frequently further single electron transfer steps. The complete electrochemical reaction vessel requires both an anode and a cathode. Only one of these electrodes, the working electrode, is involved with the chemical reaction of interest, oxidation at the anode or reduction at the cathode. The second electrode is the counter electrode and usually some simple inorganic reaction occurs here, such as hydrogen evolution if this is a cathode or oxygen evolution if this is an anode. The space between the anode and cathode is filled with an ionised salt solution and charge passes through the solution between the electrodes by migration of ions.
The simplest design of electrochemical cell has two electrodes dipping into the solution containing the substrate and the supporting electrolyte. A cell of this type is suitable for the Kolbe oxidation of carboxylate ions (see p. 316) where the anode reaction is given by Equation 1.1 and the cathode reaction is the evolution of hydrogen (Equation 1.2). Both the substrate and the hydrocarbon product are inert
towards reduction at the cathode.
For many processes, however, it is necessary to employ a divided cell in which the anode and cathode compartments are separated by a barrier, allowing the diffusion of ions but hindering transfer of reactants and products between compartments. This prevents undesirable side reactions. Good examples of the need for a divided cell are seen in the reduction of nitrobenzenes to phenylhydroxylamines (p. 379) or to anilines (p. 376). In these cases the reduction products are susceptible to oxidation and must be prevented from approaching the anode. The cell compartments can be divided with a porous separator constructed from sintered glass, porous porcelain or a sintered inert polymer such as polypropene or polytetrafluoroethene. Another type of separator uses woven polytetrafluoroethene cloth which has been exposed to a soluble silicate and dilute sulphuric acid so that silicic acid precipitates into the pores [1]. On a laboratory scale porous porcelain and sintered glass are the most commonly used materials.
On an industrial scale, ion-exchange membranes are most frequently used for the separator material [2]. Cationic and anionic types are both available and a sulphonated polytetrafluoroethene cation exchange resin, which can withstand aggressive conditions, is frequently used. Arrangements for sealing this type of separator into a laboratory scale glass cell are also available.
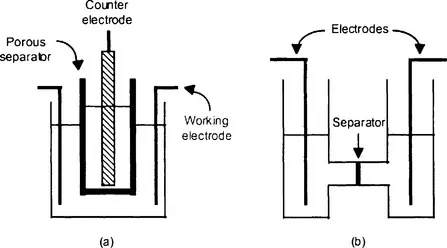
General purpose laboratory scale glass cells are either of the beaker-type (Figure 1.1a) or the H-type (Figure 1.1b). The early pioneers of organic electrochemistry used beaker-type cells, with cylindrical symmetry, and the separator was either a porous porcelain pot or a sintered glass disc [3]. Designs for beaker-type cells in more modern materials have been described [4]. The H-type cell can be designed to use either one or two sintered glass separators [5]. Oxygen must be excluded from the cathode compartment during electrochemical reduction otherwise current is consumed by the reduction of oxygen to water and the highly reactive superoxide anion is generated as an intermediate. A flow of inert gas is maintained in the cathode compartment. It is not essential to exclude oxygen during electrochemical oxidation but usually a flow of inert gas is maintained in the anode compartment so as to dilute any oxygen, which is evolved. A stirring device is necessary to decrease the thickness of the diffusion layer around the working electrode.
Figure 1.1 Cells used for laboratory scale electrochemical preparations: (a) a beaker-type cell; (b) an H-type cell.
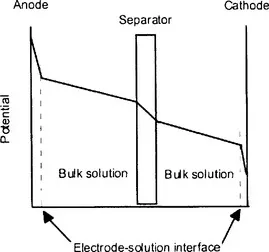
The voltage drop across a working electrochemical cell is not uniformly distributed. This is shown schematically in Figure 1.2. A large proportion is due to the electrical resistance of the electrolyte and the separator. This, of course, can be decreased by a suitable cell design. The voltage drop across the working electrode solution interface determines the rate constant for the electrochemical reaction. It is often advantageous to maintain a constant potential drop across this interface to control the rate of unwanted side reactions. The working potential is measured relative to a reference electrode and probe, placed close to the working electrode surface. An aqueous saturated calomel electrode is the most frequently used reference. The relative potentials of other reference half-cells are given in Table 1.1. The reference electrode dips into a salt bridge containing the electrolyte used in the main electrochemical cell. The salt bridge can be terminated either by a thin Luggin-Harber capillary [6] placed close to the working electrode or by a plug of porous Vycor glass [7] or an inert fibre [8]. For non-aqueous electrochemistry IUPAC recommends the ferrocene-ferricinium couple as an internal reference standard of potential [9]. It is suitable for use in linear sweep and cyclic voltammetry but not for preparative scale experiments. The couple has potentials of +0.69 and +0.72 V vs. nhe in acetonitrile and dimethylformamide respectively [10].
TABLE 1.1
Potentials of some reference electrodes relative to either the standard hydrogen electrode or the saturated calomel electrode.
| Electrochemical cell | Potential / V | Ref. |
| (Pt)/H2, H3O+ (a = 1) || KC1 (satd.) / AgCl (satd.) /Ag | 0.199 | [12] |
| (Pt)/H2, H3O+ (a = 1) || KC1 (1.0 M) / Hg2Cl2(satd.) / Hg | 0.283 | [12] |
| (Pt)/H2, H3O+ (a = 1) || KC1 (satd.)/ Hg2Cl2 (satd.)./ Hg | 0.244 | [12] |
| Aqueous sce || 0.1 M NaClO4 in CH3CN || 0.01 M AgNO3 in CH3CN / Ag | 0.253 | [13] |
| Aqueous sce || 0.1 M Et1NClO4 || Me2CHO NaCl(satd.). CdCl2 (satd) / Cd, Hg | −0.737 | [14] |
| Aqueous sce || 0.1 Mu4I in 0.1 M Bu4NI in Mc2NCHO / Agl (sat.) / Ag | −0.32 | [15] |
| Aqueous sce || 0.1 M Et1NI in Me2CHO / Agl (satd.) / Ag | −0.638 | [16] |
Further data in ref. [17].
Figure 1.2 Distribution of potential across a working electrochemical cell. The potential drop across the working electrode-solution interface drives the cell reaction.
There is a potential drop V across the solution between the layer around the working electrode and the tip of the reference probe. This is related to the separation distance d by Equation 1.3 where i is the current flowing through the c...