
- 496 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Handbook of Solid Phase Microextraction
About this book
The relatively new technique of solid phase microextraction (SPME) is an important tool to prepare samples both in the lab and on-site. SPME is a "green" technology because it eliminates organic solvents from analytical laboratory and can be used in environmental, food and fragrance, and forensic and drug analysis. This handbook offers a thorough background of the theory and practical implementation of SPME. SPME protocols are presented outlining each stage of the method and providing useful tips and potential pitfalls. In addition, devices and fiber coatings, automated SPME systems, SPME method development, and In Vivo applications are discussed.
This handbook is essential for its discussion of the latest SPME developments as well as its in depth information on the history, theory, and practical application of the method.
- Practical application of Solid Phase Microextraction methods including detailed steps
- Provides history of extraction methods to better understand the process
- Suitable for all levels, from beginning student to experienced practitioner
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1. Solid-Phase Microextraction in Perspective
Janusz Pawliszyn
Department of Chemistry, University of Waterloo, Waterloo, Ontario, Canada
The analytical procedure for complex samples consists of several steps that typically include sampling, sample preparation, separation, quantitation, statistical evaluation, and decision making. The chapter summarises the strategies leading to integration of the analytical process to increase throughput, reduce use of organic solvents and facilitate on-site analysis. Improvements in extraction techniques involved in sample preparation are identified as a key challenge to accomplish these goals. A unified approach to classification of extraction techniques based on the fundamental principles behind different extraction approaches is proposed, which leads to the rational choice of appropriate techniques for a given task. Advantages and limitations of exhaustive extraction and microextraction approaches are discussed. The significance of integration with sampling to facilitate on-site analysis, including in vivo applications, is emphasised. There are many advantages of microextractions in this regard, which can be realised to a higher or lesser degree depending on the geometric configuration of the instrument. Solid-phase microextraction is briefly introduced and compared to solid-phase extraction.
Keywords
Analytical process, complex samples, extraction, sampling, classification, miniaturisation, integration, on-site analysis, microextraction, exhaustive extraction
1.1. Sample Preparation as Part of the Analytical Process
The analytical procedure for complex samples consists of several steps that typically include sampling, sample preparation, separation, quantitation, statistical evaluation and decision making (Figure 1.1).
 |
| Figure 1.1 Steps in the analytical process. |
Each step is critical for obtaining correct and informative results. The sampling step includes deciding where to get samples that properly define the object or problem being characterised and then choosing a method to obtain samples in the right amounts. The objective of the sample preparation step is to isolate the components of interest from a sample matrix. This is because most analytical instruments cannot handle the matrix directly. Sample preparation involves extraction procedures and can also include ‘clean-up’ procedures for very complex ‘dirty’ samples. This step must also bring the analytes to a suitable concentration level for detection; therefore, sample preparation methods typically include enrichment. During the separation step of the analytical process, the isolated complex mixture containing target analytes is divided into its constituents, typically by means of chromatographic or electrophoretic techniques. Quantitation is the determination of amounts of the identified compounds. The identification can be based on a retention time combined with selective detection; more frequently, however, instruments providing more specific information (namely, mass spectrometers) are used to eliminate possible errors in quantification due to interferences. Statistical evaluation of the results provides an estimate of the target compound’s concentration in the sample being analysed. The data will then support appropriate decisions, which might include taking another sample for further investigation.
It is important to note, as emphasised in Figure 1.1, that analytical steps follow one after the other, and a subsequent step cannot begin until the preceding one has been completed. Therefore, the slowest step determines the overall speed of the analytical process, and improving the speed of a single step may not result in a throughput increase. To increase the throughput of analysis, all steps need to be considered. Also, errors performed in any preceding step, including sampling, will result in the overall poor performance of the procedure.
There have been major breakthroughs in the development of improved instrumentation, which involve miniaturisation of analytical devices and hyphenation of different steps into one system. It is recognised that an ideal instrument would perform all the analytical steps without human intervention, preferably directly on the site where an investigated system is located rather than moving the sample to the laboratory, as is currently done. This approach would eliminate the errors and the time associated with sample transport and storage and result in more accurate, more precise and faster production of analytical data. Although such a total analysis system (TAS) is challenging to build, today’s sophisticated instruments, such as the gas chromatograph/mass spectrometer (GC–MS) or liquid chromatograph/mass spectrometer (LC–MS), can separate and quantify complex mixtures and automatically apply chemometric methods to evaluate results statistically. It is much more difficult to hyphenate sampling and sample preparation steps, primarily because the current state of the art in sample preparation techniques employs multistep procedures involving organic solvents. These characteristics make it difficult to develop a method that integrates sampling and sample preparation with separation methods for the purposes of automation. The result is that more than 80% of analysis time is currently spent on sampling and sample preparation steps.
One of the reasons that progress in the area of sample preparation is so slow is that the fundamentals of extraction involving natural, frequently complex samples are much less developed and understood compared to physicochemically simpler systems used in separation and quantification steps, such as chromatography and mass spectrometry. This situation creates an impression that rational design and optimisation of extraction systems is not possible. Therefore, development of sample preparation procedures is frequently considered to be ‘art’, not ‘science’.
This situation creates a tendency by practitioners and regulatory agencies to prefer exhaustive over non-exhaustive techniques (Figure 1.2), even though this choice frequently results in labour-intensive and costly procedures. The main objective of the exhaustive techniques is to remove analytes completely from a sample matrix and transfer them to the extraction phase. The fundamental advantage of exhaustive methods is that, in principle, they do not require calibration because the vast majority of analytes are transferred to the extraction phase. There are alternative extraction techniques, however, with their own unique advantages, which have been developed to reduce solvent use and improve performance. A summary of extraction techniques is given below.
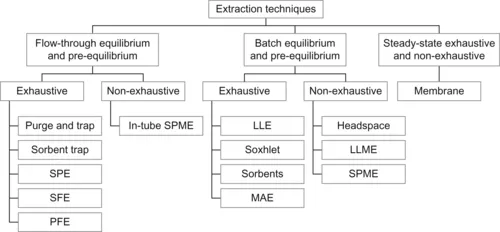 |
| Figure 1.2 Classification of extraction techniques. |
1.2. Classification of Extraction Techniques
Figure 1.2 provides a classification of extraction techniques and unifies the fundamental principles behind the different extraction approaches. In principle, exhaustive extraction approaches do not require calibration because most analytes are transferred to the extraction phase by employing overwhelming amounts of it. In practice, however, confirmation of satisfactory recoveries is implemented in the method by using surrogate standards. To reduce the amounts of solvents and time required to accomplish exhaustive removal, batch equilibrium techniques (e.g. liquid–liquid extractions) are frequently replaced by flow-through techniques.
For example, a sorbent bed can be packed with the extraction phase dispersed on a supporting material; when a sample is passed through, the analytes in the sample are retained on the bed. Large volumes of sample can be passed through a small cartridge, and the flow through the well-packed bed facilitates efficient mass transfer. The extraction procedure is followed by desorption of analytes into a small volume of solvent, resulting in substantial enrichment and concentration of the analytes. This strategy is used in sorbent-trap techniques and in solid-phase extraction (SPE). 1 Alternatively, sample (typically a solid sample) can be packed in the bed and the extraction phase can be used to remove and transport the analytes to the collection point. In supercritical fluid extraction, compressed gas is used to wash analytes from the sample matrix; an inert gas at atmospheric pressure performs the same function in purge-and-trap methods. For example, in dynamic solvent extraction in a Soxhlet apparatus, the solvent continuously removes the analytes from the matrix at the boiling point of the solvent. In more recent pressurised fluid extraction techniques, smaller volumes of organic solvent (or even water) are used to achieve greater enrichment at the same time as extraction because of the solvent’s increased capacity and elution strength at high temperatures and pressures. 2
Alternatively, non-exhaustive approaches can be designed on the basis of the principles of equilibrium, pre-equilibrium and permeation. 3 Although equilibrium non-exhaustive techniques are fundamentally analogous to equilibrium-exhaustive techniques, the capacity of the extraction phase is smaller and is usually insufficient to remove most of the analytes from the sample matrix. This is because a small volume of the extracting phase is being used relative to the sample volume, such as is employed in microextraction [solvent microextraction4 or solid-phase microextraction (SPME5)] or a low sample matrix–extraction phase distribution constant, as is typically encountered in gaseous headspace techniques. 6
Pre-equilibrium conditions are accomplished by breaking the contact between the extraction phase and the sample matrix before equilibrium with the extracting phase has been reached. Although the devices used are frequently identical to those of microextraction systems, shorter extraction times are employed. The pre-equilibrium approach is conceptually similar to the flow-injection analysis (FIA) approach, 7 in which quantification is performed in a dynamic system and system equilibrium is not required to obtain acceptable levels of sensitivity, reproducibility and accuracy. In permeation techniques, e.g. membrane extraction, 8 continuous steady-state transport of analytes through the extraction phase is accomplished by simultaneous re-extraction of analytes. Membrane extraction can be made exhaustive by designing appropriate membrane modules and optimising the sample and stripping flow conditions, 9 or it can be optimised for throughput and sensitivity in non-exhaustive, open-bed extraction. 10 Because membrane extr...
Table of contents
- Cover image
- Table of Contents
- Front-matter
- Copyright
- Dedication
- Preface
- List of Contributors
- 1. Solid-Phase Microextraction in Perspective
- 2. Theory of Solid-Phase Microextraction
- 3. Development of SPME Devices and Coatings
- 4. SPME Commercial Devices and Fibre Coatings
- 5. Automated SPME Systems
- 6. Calibration
- 7. Solid-Phase Microextraction Method Development
- 8. SPME and Environmental Analysis
- 9. Application of Solid-Phase Microextraction in Food and Fragrance Analysis
- 10. Drug Analysis by SPME
- 11. Ligand—Receptor Binding and Determination of Free Concentrations
- 12. In Vivo Sampling with Solid-Phase Microextraction
- 13. Solid-Phase Microextraction Protocols
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Handbook of Solid Phase Microextraction by Janusz Pawliszyn in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.