![]()
Chapter 1
Defining Proteins
Publisher Summary
Amino acids are the major building blocks of proteins. Proteins are both biological entities and physicochemical compounds. They exist in living organisms where they have particular functions for which they were specifically designed by evolution. The design of any such system is for the use of the organism that made the protein and not necessarily for the benefit of the hungry human population. In prepared and processed foods, proteins are often used as ingredients, for example, soy protein, milk caseinate, and egg white. The food proteins are among the less complex systems consisting merely of a nutritious collection of amino acids because of their limited biological purpose as food. Early biochemists often used these proteins because of their ready availability and easy access for study.
Can the term protein be simply defined? The word itself is ambiguous, and on different occasions different definitions must be used. If the reader is already familiar with some terminology, he or she recognizes that the problem usually hinges on whether or not amino acids alone are to be considered protein. Amino acids are certainly the major building blocks of proteins, but do they in fact “define” a protein? This choice often determines the operational definition of a protein; thus, it is important to know exactly what each experimental technique measures. The choice of technique often depends on (or should depend on) the use of the information to be obtained.
Proteins are both biological entities and physicochemical compounds, and will be examined in both contexts in this volume. We will approach their chemical and physical properties as chemists despite the fact that their use in the food supply emphasizes their biological nature. Proteins exist in living organisms where they have particular functions for which they were specifically designed by evolution. It is important to remember that the design of any such system is for the use of the organism that made the protein and not necessarily for the benefit of the hungry human population.
As food scientists we are generally interested in commodities that are no longer “living,” that is, in their postmortem or postharvest changes. Therefore, we must be aware of the biochemical changes that occur in “dead” animals after slaughter. Under many circumstances (e.g., if an animal is under stress at the time of slaughter), the conditions of the living state affect the ultimate food product. A living system can yield a product directly, without necessitating the destruction of the source; eggs, milk, and most fruits are examples. However, food scientists must be concerned with changes taking place in the product after separation from the living source.
Experimentally, we often deal with proteins in an isolated system using “purified” or partially purified preparations. The isolated system or the purified protein is not always identical to the same protein in its native state. The protein chemist must constantly decide if that which is learned about a protein in the test tube is relevant to the system from which it was derived. All proteins are essentially denatured by the time they are studied. (The word “denatured” is also vaguely defined, implying that the protein is no longer in its original or “natured,” form. Because this term has been abused in the literature, it will not be used again in this volume unless it is defined specifically.) We must limit our examination to food proteins that exist in the greatest quantities and to the enzymes of importance, that is, those that cause changes in foods. Although we will discuss enzymes when appropriate, our emphasis will be on the chemistry of the proteins themselves.
In prepared and processed foods, proteins are often used as ingredients, for example, soy protein, milk caseinate, and egg white. Thus, we are interested in the food functionality of these materials; their ability to be whipped, to form a gel, to coagulate, to bind water, to bind fat, etc. Other questions become important: How stable are the gels, emulsions, or foams that are formed? What changes occur in the proteins during processing, heating, or freezing? We must be aware that even such a simple process as homogenization may change the chemistry of a protein. In many products, the changes of interest are those involving the proteins. After foods are processed, food scientists and nutritionists must be concerned with changes in digestibility and the ability of the body to utilize the proteins, ultimately leading to the study of protein and amino acid metabolism in living organisms. Discussions of this metabolism can be found in any general biochemistry text. (See Selected Readings at end of volume for recommended additional reading.)
In our examination of the proteins of selected major food commodities, it is important to note again that many of these commodities are biologically designed as food storage systems that exist for the purpose of feeding the next generation of that species. Humans simply steal it from its intended recipient; for example, instead of allowing the egg to develop into another chicken, we take it for our own food use. Milk falls into this category along with the edible parts of many grains and legumes. Because of their limited biological purpose as food, these “food” proteins are often among the less complex systems, consisting merely of a nutritious collection of amino acids. Early biochemists often used these proteins because of their ready availability and easy access for study.
Of the edible protein foods, meat is the major exception to this pattern; it does not exist as a future food for a species. Meat is designed as muscle, a contractile system to do work and move the organism. The relation of muscle as a living biological structure to its properties as a food is of particular research interest and will be the source of many of our examples. The muscle system is one of the best studied insoluble protein systems. Traditionally, most scientists have preferred to isolate soluble proteins and work with solutions. As scientists started to examine membranes and other insoluble structures of the cell, they needed to develop methodologies to study reactions of interest that do not take place in solution. Muscle (meat) scientists have had this problem for many years. Unfortunately, the plant proteins that have recently become even more popular as food resources also present serious solubility problems.
Later chapters include a variety of chemical and physical techniques as these are used to examine proteins. The goal is that the reader will understand the techniques; this is not a laboratory manual designed to instruct the student on the step-by-step execution of these experiments. Although occasional bits of practical advice may be included, these are not the main focus of the discussion of techniques.
One of our aims is that the reader be able to evaluate and use the results of other researchers. To understand the validity of a measurement, one must understand the technique: What does the technique measure? How does the instrument work? Was it used properly? We must all make decisions in our own work based on what we have read in the literature. Unfortunately, some of the literature is inadequate for current needs; it is not solidly based experimentally, or contains too many flaws, or lacks too much of the information needed to understand what was actually done. If we can improve the reader’s ability to evaluate published information more critically, he or she will be better prepared to make the kinds of judgments required of a food scientist.
Throughout this volume, we will analyze carefully the rationale for determining which tests to use to obtain specific desired information. An example is the series of questions related to the problem, How do we measure protein? Should we measure protein by doing a Kjeldahl, by using the Lowry method, or by a spectral method? Must we precipitate with trichloroacetic acid (TCA) first? It is necessary to start with the specific aim of the series of experiments: Why is the measurement required? What is the purpose of the data? (Analytical techniques for measuring protein are discussed in detail in Chapter 10.)
Another goal is to extend the reader’s protein chemistry vocabulary. Perhaps one of the other new terms will be more easily definable. And finally, the development of scientific knowledge, and the need to develop professional skepticism, are emphasized.
PROBLEM SET
1. What is a protein? The definition should indicate what material(s) you would term protein(s) and perhaps some closely related materials you would not include.
2. What are some of the strengths and weaknesses of your definition? (Note borderline cases that must be included or excluded arbitrarily.) What differences might be expected for “protein” definitions in chemistry, food science, and nutrition (human and animal)?
[These questions will be asked again at the end of the text. Please save your answer for future comparison.]
![]()
Chapter 2
Proteins as the Essence of Life
Publisher Summary
Proteins are involved in body structure; they make up the muscle system, which constitutes more than 50% of the human body and are involved in various other forms of movement. Cellular streaming, mitosis, meiosis, and cell splitting involve proteins resembling those of the contractile systems. Proteins are a part of the structure of chromosomes and are responsible for their movement. Traditionally, the nucleic acid content of chromosomes has been emphasized; however, it is the histones, as a part of the system of regulators, that turn transcription on and off. Chromosome translation occurs on ribosomes that are composed of both proteins and nucleic acids. The immunological defense system is also a protein system. Proteins are composed of different amino acids. A particular protein tends to have a relatively discrete molecular weight. In contrast to a carbohydrate polymer, for example, a given protein exists as many molecules with the same fixed amino acid composition.
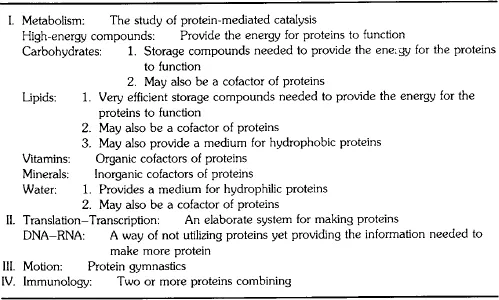
Proteins are essential to life as we know it. Table 2-I illustrates the visions of a food scientist in a protein-centered universe. We can, of course, list the functions of proteins in a more traditional manner. First, all enzymes are essentially protein, and enzymes are responsible for the chemical, mechanical, and electrical energy producing the functions of life.
TABLE 2-I
BIOLOGICAL MATERIALS AND PROCESSES AS SEEN BY A PROTEIN CHEMIST
Proteins are involved in body structure; they make up the muscle system which constitutes more than 50% of the human body (dry weight) and are involved in various other forms of movement. It now appears that cellular streaming, mitosis, meiosis, and cell splitting involve pro...