
Microbial Glycobiology
Structures, Relevance and Applications
- 1,036 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Microbial Glycobiology
Structures, Relevance and Applications
About this book
This book presents in an easy-to-read format a summary of the important central aspects of microbial glycobiology, i.e. the study of carbohydrates as related to the biology of microorganisms. Microbial glycobiology represents a multidisciplinary and emerging area with implications for a range of basic and applied research fields, as well as having industrial, medical and biotechnological implications.- Individual chapters provided by leading international scientists in the field yield insightful, concise and stimulating reviews- Provides researchers with an overview and synthesis of the latest research- Each chapter begins with a brief 200 word Summary/Abstract detailing the topic and focus of the chapter, as well as the concepts to be addressed- Allows researchers to see at a glance what each chapter will cover- Each chapter includes a Research Focus Box- Identifies important problems that still need to be solved and areas that require further investigation
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Otto Holst, Anthony P. Moran and Patrick J. Brennan
Summary
1. Introduction – The Bacterial Cell Envelope Encountering Environmental Challenges
2. The Gram-Negative Cell Envelope
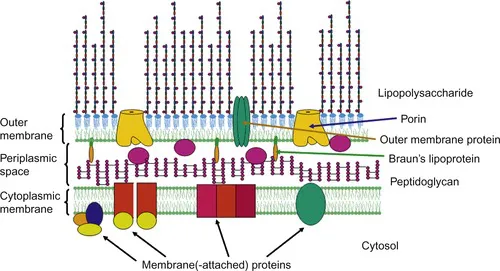 |
| Figure 1.1 A schematic model of the Gram-negative cell envelope as present in E. coli. The cell envelope comprises the cytoplasmic membrane, which is a symmetric membrane consisting mainly of phospholipids in both leaflets, the periplasmic space and the outer membrane. Embedded in the cytoplasmic membrane, i.e. membrane-attached at the cytosolic leaflet or integrated into the membrane, are a number of proteins including those important for lipopolysaccharide or capsular polysaccharide biosynthesis and transport to the periplasmic space. The thin peptidoglycan layer represents the major constituent of the periplasmic space, but which also contains proteins including transport-proteins (indicated by the ovoid shapes in the periplasm). In E. coli, Braun’s lipoprotein is present which is covalently bound to the peptidoglycan and anchored by its lipid moiety in the inner leaflet of the outer membrane. In contrast to the cytoplasmic membrane, the outer membrane represents an asymmetric membrane, i.e. comprising phospholipids in the inner and lipopolysaccharides in the outer leaflet. It contains a number of outer membrane proteins, including the porins that are water-filled channels important for the import of small molecules, like sugars or ions. Not shown are capsular polysaccharides and the enterobacterial common antigen which may also be anchored in the outer leaflet by a lipid structure. In addition, S-layer glycoproteins may be present in certain Gram-negative bacterial species (not shown). |
Table of contents
- Cover image
- Table of Contents
- Copyright
- Dedication
- List of Contributors
- Preface
- Chapter 1. Overview of the glycosylated components of the bacterial cell envelope
- Chapter 2. Bacterial cell envelope peptidoglycan
- Chapter 3. Core region and lipid A components of lipopolysaccharides
- Chapter 4. O-Specific polysaccharides of Gram-negative bacteria
- Chapter 5. Teichoic acids, lipoteichoic acids and related cell wall glycopolymers of Gram-positive bacteria
- Chapter 6. Bacterial capsular polysaccharides and exopolysaccharides
- Chapter 7. Bacterial surface layer glycoproteins and “non-classical” secondary cell wall polymers
- Chapter 8. Glycosylation of bacterial and archaeal flagellins
- Chapter 9. Glycosylated components of the mycobacterial cell wall
- Chapter 10. Glycoconjugate structure and function in fungal cell walls
- Chapter 11. Cytoplasmic carbohydrate molecules
- Chapter 12. Glycosylated compounds of parasitic protozoa
- Chapter 13. Analytical approaches towards the structural characterization of microbial wall glycopolymers
- Chapter 14. Single-molecule characterization of microbial polysaccharides
- Chapter 15. Viral surface glycoproteins in carbohydrate recognition
- Chapter 16. Biosynthesis of bacterial peptidoglycan
- Chapter 17. Biosynthesis and membrane assembly of lipid A
- Chapter 18. Biosynthesis of O-antigen chains and assembly
- Chapter 19. Biosynthesis of cell wall teichoic acid polymers
- Chapter 20. Biosynthesis and assembly of capsular polysaccharides
- Chapter 21. Biosynthesis of the mycobacterial cell envelope components
- Chapter 22. Biosynthesis of fungal and yeast glycans
- Chapter 23. Chemical synthesis of bacterial lipid A
- Chapter 24. Chemical synthesis of the core oligosaccharide of bacterial lipopolysaccharide
- Chapter 25. Chemical synthesis of lipoteichoic acid and derivatives
- Chapter 26. Chemical synthesis of parasitic glycoconjugates and phosphoglycans
- Chapter 27. Bacterial lectin-like interactions in cell recognition and adhesion
- Chapter 28. Lectin-like interactions in virus–cell recognition
- Chapter 29. Sialic acid-specific microbial lectins
- Chapter 30. Bacterial toxins and their carbohydrate receptors at the host–pathogen interface
- Chapter 31. Toll-like receptor recognition of lipoglycans, glycolipids and lipopeptides
- Chapter 32. NOD receptor recognition of peptidoglycan
- Chapter 33. Microbial interaction with mucus and mucins
- Chapter 34. Mannose–fucose recognition by DC-SIGN
- Chapter 35. Host surfactant proteins in microbial recognition
- Chapter 36. T-Cell recognition of microbial lipoglycans and glycolipids
- Chapter 37. Extracellular polymeric substances in microbial biofilms
- Chapter 38. Physicochemical properties of microbial glycopolymers
- Chapter 39. Microbial biofilm-related polysaccharides in biofouling and corrosion
- Chapter 40. Microbial glycosylated components in plant disease
- Chapter 41. Antigenic variation of microbial surface glycosylated molecules
- Chapter 42. Phase variation of bacterial surface glycosylated molecules in immune evasion
- Chapter 43. Molecular mimicry of host glycosylated structures by bacteria
- Chapter 44. Role of microbial glycosylation in host cell invasion
- Chapter 45. Exopolysaccharides produced by lactic acid bacteria in food and probiotic applications
- Chapter 46. Industrial exploitation by genetic engineering of bacterial glycosylation systems
- Chapter 47. Glycomimetics as inhibitors in anti-infection therapy
- Chapter 48. Bacterial polysaccharide vaccines
- Chapter 49. Immunomodulation by zwitterionic polysaccharides
- Chapter 50. Future potential of glycomics in microbiology and infectious diseases
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app