
eBook - ePub
Computational Systems Biology
From Molecular Mechanisms to Disease
This is a test
- 548 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
This comprehensively revised second edition of Computational Systems Biology discusses the experimental and theoretical foundations of the function of biological systems at the molecular, cellular or organismal level over temporal and spatial scales, as systems biology advances to provide clinical solutions to complex medical problems. In particular the work focuses on the engineering of biological systems and network modeling.
- Logical information flow aids understanding of basic building blocks of life through disease phenotypes
- Evolved principles gives insight into underlying organizational principles of biological organizations, and systems processes, governing functions such as adaptation or response patterns
- Coverage of technical tools and systems helps researchers to understand and resolve specific systems biology problems using advanced computation
- Multi-scale modeling on disparate scales aids researchers understanding of dependencies and constraints of spatio-temporal relationships fundamental to biological organization and function.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Computational Systems Biology by Andres Kriete,Roland Eils in PDF and/or ePUB format, as well as other popular books in Computer Science & Bioinformatics. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Introducing Computational Systems Biology
Roland Eilsa,b and Andres Krietec, aDivision of Theoretical Bioinformatics (B080), German Cancer Research Center (DKFZ), Heidelberg, Germany, bDepartment for Bioinformatics and Functional Genomics, Institute for Pharmacy and Molecular Biotechnology (IPMB) and BioQuant, Heidelberg University, Heidelberg, Germany, cSchool of Biomedical Engineering, Science and Health Systems, Drexel University, Philadelphia, PA, USA
We need to turn data into knowledge and we need a framework to do so.S. Brenner, 2002.
1 Prologue
The multitude of the computational tools needed for systems biology research can roughly be classified into two categories: system identification and behavior analysis (Kitano 2001). In molecular biology, system identification amounts to identifying the regulatory relationships between genes, proteins, and small molecules, as well as their inherent dynamics hidden in the specific kinetic and binding parameters. System identification is arguably one of the most complicated problems in science. While behavior analysis is solely performed on a model, model construction is a process tightly connected to reality but part of an iterative process between data analysis, simulation, and experimental validation (Figure 1.1). A typical modeling cycle begins with a reductionist approach, creating the simplest possible model. The modeling process generates an understanding of the underlying structures, and components are represented graphically with increasing level of formalization, until they can be converted into a mathematical representation. The minimal model then grows in complexity, driven by new hypotheses that may not have been apparent from the phenomenological descriptions. Then, an experiment is designed using the biological system to test whether the model predictions agree with the experimental observations of the system behavior. The constitutive model parameters may be measured directly or may be inferred during this validation process, however, the propagation of errors through these parameters present significant challenges for the modeler. If data and predictions agree, a new experiment is designed and performed. This process continues until sufficient experimental evidence in favor of the model is collected. Once the system has been identified and a model constructed, the system behavior can be studied, for instance, by numerical integration or sensitivity analysis against external perturbations.
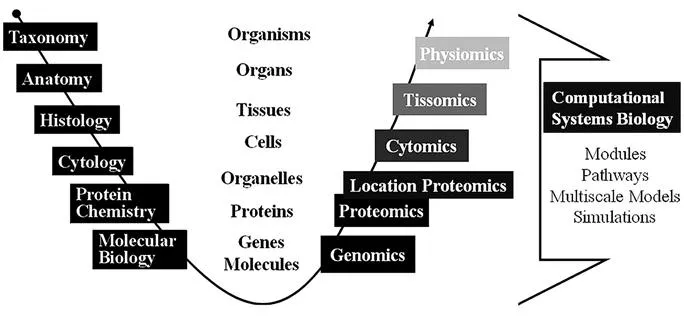
Although the iterative process is well defined, the amount of data to be merged into this process can be immense. The human genome project is one of the hallmarks indicating a turn from a reductionistic approach in studying biological systems at increasing level, into a discovery process using high-throughput techniques (Figure 1.2). Ongoing research increases the wealth of contemporary biological information residing in some thousand public databases providing descriptive genomics, proteomics and enzyme information, gene expression, gene variants and gene ontologies. Refined explorative tools, such as new deep sequencing, along with the emergence of new specialized -omics (metabolomics, lipidomics, pharmacogenomics) and phenotyping techniques, constantly feed into this data pool and accelerate its growth.
Given the enormous and heterogeneous amount of data, computational tools have become indispensable to mine, analyze, and connect such information. The aggregate of statistical bioinformatics tools to collect, store, retrieve, visualize, and analyze complex biological data has repeatedly proven useful in biological decision support and discovery. Deciphering the basic building blocks of life is a necessary step in biological research, but provides only limited knowledge in terms of understanding and predictability. In the early stages the human genome project stirred the public expectation for a rapid increase in the deciphering of disease mechanisms, more effective drug development and cure. However, it is well recognized that the battery of mechanisms involved in the proliferation of complex diseases like cancer, chronic diseases, or the development of dementias cannot be understood solely on the basis of knowing all its molecular components.
As a consequence, a lack of system level understanding of cellular dynamics has prevented a substantial increase in the number of new drugs available for treatment, drug efficacy, or eradication of any specific diseases. In contrast, pharmaceutical companies are currently lacking criteria to select the most valuable targets, R&D expenses skyrocket, and new drugs rarely hit the market and often fail in clinical trials, while physicians face an increasing wealth of information that needs to be interpreted intelligently and holistically.
Analysis of this dilemma reveals primary difficulties due to the enormous biomolecular complexity, structural and functional unknowns in a large portion of gene products and a lack of understanding of how the concert of molecular activities transfers into physiological alterations and disease. It has been long recognized that the understanding of cells as open systems, interacting with the environment, performing tasks and sustain homeostasis, or better homeodynamics (Yates 1992), requires the development of foundations for a general systems theory that started with the seminal work of Bertalanffy (Von Bertalanffy 1969). It appears that with the ever increasing quality and quantity of molecular data, mathematical models of biological processes are even more in demand. For instance, an envisioned blueprint of complex diseases will not solely consist of descriptive flowcharts as widely found in scientific literature or in genomic databases. They should rather be based on predictive, rigorously quantitative data-based mathematical models of metabolic pathways, signal transduction cascades, cell-cell communication, etc. The general focus of biomedical research on complex diseases needs to change from a primarily steady-state analysis at the molecular level to a systems biology level capturing the characteristic dynamic behavior. Such biosimulation concepts will continue to transform current diagnostic and therapeutic approaches to medicine.
2 Overview of the content
This completely revised, second edition of this book presents examples selected from an increasingly diverse field of activities, covering basic key methods, development of tools, and recent applications in many complex areas of computational systems biology. In the following, we will broadly review the content of the chapters as they appear in this book, along with specific introductions and outlooks.
The first section of this book introduces essential foundations of systems biology, principles of network reconstruction based on high-throughput data with the help of engineering principles such as control theory. Robert B. Russell, Gordana Apic, Olga Kalinina, Leonardo Trabuco, Matthew J. Betts, and Qianhao Lu provide an introduction (Chapter 2) on “Structural Systems Biology: modeling interactions and networks for systems studies.” Molecular mechanisms provide the most detailed level for a mechanistic understanding of biological complexity. The current challenges of a structural systems biology are to integrate, utilize, and extend such knowledge in conjunction with high-throughput studies. Understanding the mechanistic consequences of multiple alterations in DNA variants, protein structures, and folding are key tasks of structural bioinformatics.
Principles of protein interactions in pathways and networks are introduced by Hans V. Westerhoff, Fei He, Ettore Murabito, Frédéric Crémazy, and Matteo Barberis in Chapter 3. Their contribution is entitled “Understanding principles of the dynamic biochemical networks of life through systems biology” and discusses a number of basic, more recent and upcoming discoveries of network principles. The contributors review analytical procedures from flux balance in metabolic networks to measures of robustness.
In Chapter 4, Ursula Klingmüller, Marcel Schilling, Sonja Depner, and Lorenza A. D‘Alessandro review the “Biological foundations of signal transduction and aberrations in disease.” Signaling pathways process the external signals through complex cellular networks that regulate biological functions in a context-dependent manner. The authors identify the underlying biological mechanisms influential for signal transduction and introduce the mathematical tools essential to model signaling pathways and their disease aberrations in a quantitative fashion.
Further acceleration of progress in pathway reconstruction and analysis is contingent on the solution of many complexities and new requirements, revolving around the question of how high-throughput experimental techniques can help to accelerate reconstruction and simulation of signaling pathways. This is the theme of the review in Chapter 5 by Christina Kiel and Luis Serrano on the “Complexities underlying a quantitative systems analysis of signaling networks.” Chapter 6 by Seiya Imoto, Hiroshi Matsuno, Satoru Miyano presents “Gene networks: estimation, modeling and simulation.” The authors describe how gene networks can be reconstructed from microarray gene expression data, which is a contemporary problem. They also introduce software tools for modeling and simulating gene networks, which is based on the concept of Petri nets. The authors demonstrate the utility for the modeling and simulation of the gene network for controlling circadian rhythms.
Section 2 provides an overview of methods, mathematical tools, and examples for modeling approaches of dynamic systems. “Standards, platforms, and applications,” as presented by Herbert Sauro and Stanley Gu in Chapter 8, reviews the trends in developing standards indicative of increasing cooperation within the systems biology community, which emerged in recent years permitting collaborative projects and exchange of models between different software tools. “Databases for systems biology,” as reviewed in Chapter 9 by Juergen Eils, Elena Herzog, Baerbel Felder, Christian Lawerenz and Roland Eils provide approaches to integrate information about the responses of biological system to genetic or environmental perturbations. As researchers try to solve biological problems at the level of entire systems, the very nature of this approach requires the integration of highly divergent data types, and a tight coupling of three general areas of data generated in systems biology: experimental data, elements of biological systems, and mathematical models with the derived simulations. Chapter 10 builds on a classical mathematical modeling approach to study patterns of dynamic behaviors in biological systems. “Computational models for circadian rhythms - deterministic versus stochastic approaches,” Jean-Christophe Leloup, Didier Gonze and Albert Goldbeter demonstrates how feedback loops give rise to oscillatory behavior and how several results can be obtained in models which possess a minimum degree of complexity. Circadian rhythms provide a particular interesting case-study for showing how computational models can be used to address a wide range of issues extending from molecular mechanism to physiological disorders.
Reinhard Laubenbacher and Pedro Mendes review “Top-down dynamical modeling of molecular regulatory networks,” Chapter 11. The modeling framework discussed in this chapter considers mathematical methods addressing time-discrete dynamical systems over a finite state set applied to decipher gene regulatory networks from experimental data sets. The assumptions of final systems states are not only a useful modeling concept, but also serve an explanation of fundamental organization of cellular complexities. Chapter 12, entitled “Multistability and multicellularity: cell fates as high-dimensional attractors of gene regulatory networks,” by Joseph X. Zhou and Sui Huang, investigates how the high number of combinatorially possible expression configurations collapses into a few configurations characteristic of observable cell fates. These fates are proposed to be high-dimensional attractors in gene activity state space, and may help to achieve one of the most desirable goal of computational systems biology, which is the development of whole cell models. In Chapter 13 John Cole, Mike J. Hallock, Piyush Labhsetwar, Joseph R. Peterson, John E. Stone, and Zaida Luthey-Schulten review “Whole cell modeling strategies for single cells and microbial colonie...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributors
- Preface
- Chapter 1. Introducing Computational Systems Biology
- Chapter 2. Structural Systems Biology: Modeling Interactions and Networks for Systems Studies
- Chapter 3. Understanding Principles of the Dynamic Biochemical Networks of Life Through Systems Biology
- Chapter 4. Biological Foundations of Signal Transduction, Systems Biology and Aberrations in Disease
- Chapter 5. Complexities in Quantitative Systems Analysis of Signaling Networks
- Chapter 6. Gene Networks: Estimation, Modeling, and Simulation
- Chapter 7. Reconstruction of Metabolic Network from Genome Information and its Structural and Functional Analysis
- Chapter 8. Standards, Platforms, and Applications
- Chapter 9. Databases, Standards, and Modeling Platforms for Systems Biology
- Chapter 10. Computational Models for Circadian Rhythms: Deterministic versus Stochastic Approaches
- Chapter 11. Top-Down Dynamical Modeling of Molecular Regulatory Networks
- Chapter 12. Discrete Gene Network Models for Understanding Multicellularity and Cell Reprogramming: From Network Structure to Attractor Landscapes Landscape
- Chapter 13. Stochastic Simulations of Cellular Processes: From Single Cells to Colonies
- Chapter 14. Advances in Machine Learning for Processing and Comparison of Metagenomic Data
- Chapter 15. Systems Biology of Infectious Diseases and Vaccines
- Chapter 16. Computational Modeling and Simulation of Animal Early Embryogenesis with the MecaGen Platform
- Chapter 17. Developing a Systems Biology of Aging
- Chapter 18. Molecular Correlates of Morphometric Subtypes in Glioblastoma Multiforme
- Chapter 19. Applications in Cancer Research: Mathematical Models of Apoptosis
- Author Index
- Subject Index