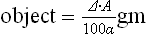(1) Phase Contrast and Interference Microscopy.
The need to study living cells is becoming increasingly important and the conventional optical microscope has severe limitations in this respect, as indeed, has the human eye. Most living cells are virtually transparent objects whose constituent parts differ but little from one another in refractive index, so that there is hardly any visible contrast between them. Moreover, the refractive index of most living cells is very close to that of the aqueous media in which they are likely to be placed for microscopical examination. In consequence living cytoplasm appears structureless and empty to a large degree. This is, of course, one of the reasons why, in the past, so much use was made of cells that had been chemically treated (fixed) and coloured with various dyes, because this method revealed structures that could not be seen in the living cell. It was often open to question, however, whether such structures were artifacts of the technique rather than objects actually present. The great advantage of phase contrast microscopy is that it introduces contrast by optical means into unstained preparations of transparent objects e.g. living cells. A mathematical treatment of the theory of phase contrast is complex, but the following general account will serve to introduce the principle of the method.
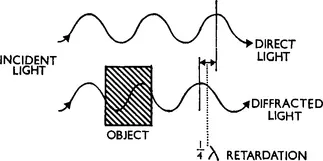
When light passes through a transparent object which is mounted in a medium which differs in refractive index from itself, it does so with no change in the amplitude of the light waves. Since the retina of the eye is sensitive only to amplitude changes the object is therefore virtually invisible. Although there has been no change in the amplitude of the transmitted light it has nevertheless been affected by its passage through the object. Diffraction generates a set of new waves which differ in phase from the direct light which has not passed through the object (Fig. 1.1). This change in the phase of the diffracted light is of the order of a quarter of a wavelength (½λ) for a typical cell.
FIG. 1.1 A diagram of the effect of a transparent object on light. The waves which have passed through it are retarded in phase by about ¼λ with respect to the original beam but their amplitude is unchanged.
The microscope image is formed as a result of interference between the direct light and the light diffracted by the object, so that if the phase difference between these two sets of waves can be further increased by optical means until the total difference between them is about ½λ, then maximal destructive interference will result. There will thus be formed amplitude differences which can be recognized by the eye. The transparent object on the stage of the microscope thus is seen as if it were an absorbing object and it appears dark to a greater or lesser degree, depending on the amount of phase change which it has introduced into the diffracted light.
The practical problem, clearly, is to devise an optical system whereby the incident and diffracted waves are separated and the phase difference between them increased by ½λ. This was achieved many years ago by Zernike but the significance of his discovery was not immediately appreciated by biologists.
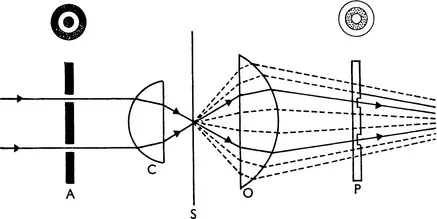
Fig. 1.2A shows the optical system of the phase contrast microscope. An opaque diaphragm (A) containing a transparent annulus is placed at the back focal plane of the substage condenser (C). Direct light, unaffected by the object on the stage at S, will form an image of the annulus in the back focal plane of the objective (O); the diffracted light which appears to emanate from the object will, however, be evenly spread over the same area. Inside the objective a transparent phase plate (P) which has a circular groove in it is mounted so that the direct image of the annulus (A) coincides exactly with the groove.
FIG. 1.2A (top) The principle of the phase-contrast microscope; A represents the illuminating annulus, C the condenser, S the plane of the specimen, O the microscope objective lens and P the phase plate situated in the back focal plane of the objective. The path of the diffracted light is shown dotted, that of the direct light in a full line.
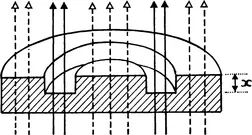
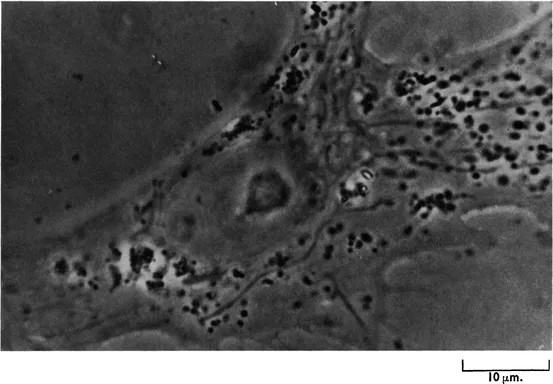
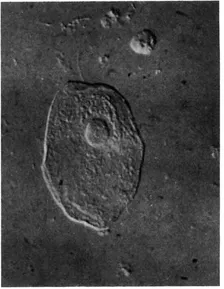
The diffracted light will pass through the full thickness of the phase plate both inside and outside the groove, whilst the direct light passes through the thinner area occupied by the groove. The extra thickness of the glass (x in Fig. 1.2B) that the diffracted light has to traverse is so calculated that it introduces a further ¼λ phase change relative to the direct light. The resulting destructive interference will produce a final image in which considerable detail can be seen (e.g. nucleus, chromosomes, mitochondria, lipid droplets, etc.). Fig. 1.3 shows the appearance of a living fibroblast visualized by phase contrast microscopy.
FIG. 1.2B (below) A diagram of the phase plate in the objective. Note that the direct light all passes through the groove in the plate, whilst the diffracted light suffers a further retardation by having to pass through the extra thickness “x”.
FIG. 1.3 A living fibroblast in culture photographed with the phase contrast microscope. Note the “halo” around the cell border and the high contrast of the cytoplasmic inclusions. The mitochondria may be seen as long filamentous objects.
When a living cell is immersed in a medium whose refractive index is the same as its own it shows minimum contrast, that is it becomes invisible in the phase microscope. Now, many cells will survive quite well in osmotically-balanced aqueous solutions of proteins such as bovine plasma albumin and it is not difficult to find, by trial and error, that solution of known concentration which causes a particular cell (e.g. an erythrocyte, a fibroblast, etc.) to become virtually invisible when immersed in it.
Under these conditions the concentration of protein in the mounting medium will be approximately equal to that of the proteins in the cell. Since, in some cells, there may be significant amounts of other substances (e.g. carbohydrates) in addition to protein, more precisely the value obtained indicates the concentration of protoplasmic solids. Where this method of cell refractometry has been used the results have been in good agreement with those obtained by using other and quite different methods. Phase contrast microscopy has the great advantage that high contrast images of living cells can be achieved easily and with relatively simple equipment; because of the incomplete separation of direct and diffracted light, however, the image suffers from certain defects, especially from the presence of a marked “halo” around the edges where there is a sharp difference in refractive indices.
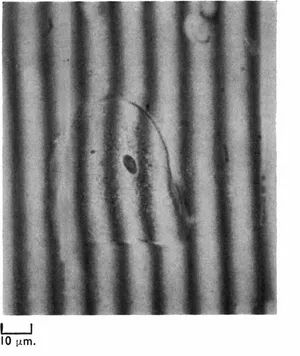
Like the phase contrast microscope, the interference microscope may be used qualitatively to provide contrast in transparent objects and quantitatively to obtain information about the concentration of substances in cells and in addition to measure cell dry mass. In all types of interference microscope a beam of coherent light (i.e. light from a single source) is split in such a way that one part of the beam passes through the specimen and the other passes to the side of it. The light which bypasses the object is called the reference beam. Before entering the objective lens the two beams are recombined by a special system called a beam recombiner. One type of system is so arranged that the field of view has distributed across it a series of parallel dark interference bands, each band representing a zone of maximum destructive interference between the two beams at recombination. Where there is no object in the field the interference bands are evenly spaced and are separated by intervals representing one wavelength (λ) (see Fig. 1.4A); when however a specimen, such as a cell, is introduced, the bands are displaced by varying amounts (see Fig. 1.4B). The degree of displacement can be measured at different points in the specimen and an average value obtained. If the displacement is D, and the distance between the bands outside the specimen is d, then D/dλ (where λ for green light = 5,460 × 10−8 cm.) corresponds to a function known as the optical path difference or Δ.
FIG. 1.4A (top left) A buccal epithelial cell photographed with the Leitz interference microscope, arranged for fringe-field working; note how the bands are displaced where they pass through the cell. × 40 objective.
FIG. 1.4B (below) A diagram of 1.4A to show how the displacement of the bands is measured and the value of D/d obtained.
It can be shown that the dry mass (
m) of an
. where
A is the area in cm
2 and α is a constant, known as the specific refraction increment;
m can therefore be calculated since the interference microscope gives the value of Δ,
A can be measured by projection and α is known.
Recently interference microscopes have become available which use a principle of beam separation devised by Nomarski. Linearly polarized light is divided into reference and object beams by means of a double quartz wedge, the Wollaston prism, placed above the condenser. A second Wollaston prism above the microscope objective effects the recombination of the two beams. Images produced by a Nomarski interference microscope have a pseudo stereoscopic or “3-D” effect in addition to the usual interference colour contrast. The “3-D” effect is given by the appearance of a dark shadow on one side of ref...