
eBook - ePub
Bleaching and Purifying Fats and Oils
Theory and Practice
Gary R. List
This is a test
- 505 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Bleaching and Purifying Fats and Oils
Theory and Practice
Gary R. List
Book details
Book preview
Table of contents
Citations
About This Book
Since the original publication of this book in 1992, the bleaching process has continued to attract the attention of researchers and the edible-oil industry. In this 2nd edition, the reader is directed to more modern techniques of analysis such as flame-atomic adsorption, graphite furnace atomic adsorption, and atomic emission spectrometry involving direct current plasma (DCP) and inductively coupled plasma (ICP). It also discusses the Freundlich Equation and reports on high-temperature water extraction, high- temperature oxidative aqueous regeneration, and extraction with supercritical CO2. Finally, various degumming methods improved over the past several decades are discussed
- Second edition features the progress in the bleaching and purifying of fats and oils since the mid-1990s
- Includes extensive details on the adsorptive purification of an oil prior to subsequent steps in the process, including refining and deodorization
- Offers practical considerations for choosing membranes, filtration equipment, and other key economic consideratons
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Bleaching and Purifying Fats and Oils by Gary R. List in PDF and/or ePUB format, as well as other popular books in Tecnología e ingeniería & Ciencia de los alimentos. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Basic Components and Procedures
H.B.W. Patterson
The Nature of Fats and Oils
In the Preface, bleaching and purifying are shown to be forms of separation of unwanted minor components from oils which, ultimately, can mean the destruction of some of them. One must recognize that the method chosen must avoid damaging the oils themselves and, if possible, their beneficial minor components. Constraints on procedure are therefore inevitable, and compromises may have to be made: these are closely related to the intended use of the final product—whether edible or technical. Finally, one must always keep in mind the relative costs of different methods, as compared to the technical gains in quality achieved (James, 1958). Some description of the chemical and physical characteristics of fats and oils and of their common minor components will make any future discussion of the methods of bleaching or purifying more readily understandable.
Triglycerides
The basic unit of a fat consists of a molecule of glycerol combined with three molecules of fatty acid. When all the fatty acid molecules are of the same kind, the result is described as a simple triglyceride; if more than one kind is present, it is a mixed triglyceride (Fig. 1.1).
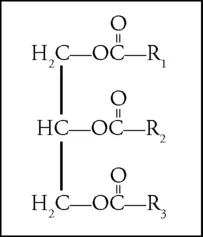
When the melting point of a triglyceride is below ambient temperature, it is commonly called an oil, and if above, a fat. The same material may be referred to as one or the other depending on the zone in which it is being handled. Various classes of fatty acids exist, and have a marked influence on the triglyceride in which they occur. In a mixed triglyceride, the sequence in which different fatty acids are distributed over positions 1, 2, and 3 has an influence on its character, especially its melting behavior (Sonntag, 1979; Taylor, 1973). Positions 1 and 3 are slightly more exposed to chemical attack than position 2 (Drozdowski, 1977; Kaimal & Lakshminarayana, 1979; Paulose et al., 1978; Sebedio et al., 1981). These matters are not important in regard to bleaching. The size of the triglyceride molecule is 1.5–2.0 nm (15–20 Å, 0.0015–0.002 μmL), so it can pass readily into meso- and macropores (see Use of Carbon) of an adsorbent, but not into micropores (under 2 nm in width). When fats are partially hydrolyzed, mono- and diglycerides result. These are important for industrial use as emulsifiers, but normally are present in crude oils in very small amounts. Just as differing fatty acids markedly affect the character of an individual triglyceride in which they occur, so the presence of differing triglycerides affects the character of a natural oil or fat, which often contains several varieties.
Fatty Acids
When a hydrocarbon chain is oxidized, terminally, so as to contain a carboxylic group,
CH3-CH2—CH2CH2COOH
the product is described as a fatty acid because many varieties of such acids occur in fats. The three simplest acids, formic, acetic and proprionic, are not included since they do not show the immiscibility-with-water characteristic of higher members. Butyric acid (CH3-CH2-CH2-COOH) is included only because it is found combined with butter. From the six-membered carbon chain (caproic acid), immiscibility with water grows as the chain lengthens. Because of the mechanism of their biochemical synthesis, the quantity of fatty acids containing an even number of carbon atoms in an unbranched chain vastly exceeds the total amount of other types (Taylor, 1973). However, because analytical techniques have improved since the 1950s, small amounts of many fatty acids with branched chains and chains containing an odd number of carbon atoms have come to light. For example, we may have a fatty acid with a total of 18 carbon atoms but containing a straight chain of 17 carbon atoms and an additional methyl group at one point or another along its length. The structural variants are positional isomers of one another. The 17-carbon atom unbranched-chain fatty acid, heptadecanoic (margaric) acid, occurs widely in animal fats, such as tallow, in very small amounts (around 1%), but is virtually absent from vegetable oils. Besides these, fatty acids were identified which contain epoxy, keto and hydroxy groups, and some which include in their chain three- (propenoid) and five- (furanoid) membered rings (Sonntag, 1979). These minor varieties usually account for less than 1% of the amount of fatty acid in common fats; occasionally a rare seed oil or fish-liver oil is found in which over one-half of the fatty acids are of exceptional types.
Saturated Fatty Acids
When all carbons in the chain hold their full complement of hydrogen, the fatty acids are saturated. These are the most stable fatty acids; whether free or in combination, their molecules pack together when solid more easily because of their regular contour. This causes a higher melting point, and as chain length increases, so does the melting point. The melting point of a fatty acid with an uneven number of carbon atoms in the chain is slightly lower than that of the even-numbered one immediately preceding it. The hydrophobic character increases with chain length. This means their sodium salts (soaps) become less soluble, but will form a more stable lather. Lauric (C12), palmitic (C16), and stearic (C18) acids are the most common saturated fatty acids (SFAs); chain lengths of up to 32 carbon atoms are found. SFAs are the most resistant to oxidation and other forms of chemical attack. They are not open to the same form of destabilization when heated with activated earth as may occur with fatty acids where considerable unsaturation is present in their composition.
Unsaturated Fatty Acids
If a hydrogen atom is missing from each of two adjoining carbon atoms in the fatty acid chain, a double bond forms between them. Those fatty acids which contain only one such double bond are described as monounsaturated. Double bonds are potential points of oxidation and other forms of chemical attack; the vulnerability increases rapidly as the number of double bonds increases. Double bonds introduce an uneven feature into the chain. When the remaining hydrogen atoms on the two adjoining carbon atoms lie on the same side of the chain, the latter is seen as assuming an arc at that point, with the two hydrogen atoms lying toward the outside of the arc; this condition or isomer is known as a cis double bond. When the remaining two hydrogen atoms lie on opposite sides of the chain, a slight kink or dog-leg effect arises and is described as the trans isomer. These two forms exist because the rotational freedom of a single bond is lost, and the double bond now introduces a restriction or spatial rigidity.
Unsaturation decreases hydrophobic character in comparison with a SFA, but as with the latter, an increase in chain length increases the hydrophobic effect. The trans isomeric form of fatty acid has the higher melting point, seemingly because the carbon chains pack together more easily to form a stable structure in the solid state. Cis isomers are associated with softness or liquidity, and this form is markedly dominant in the natural unsaturated fats and oils. Cis and trans forms are geometric isomers. If the double bond is located at different positions in the chain, these are positional isomers, and they also may have distinctly different physical characteristics, as Fig. 1.2 makes clear.
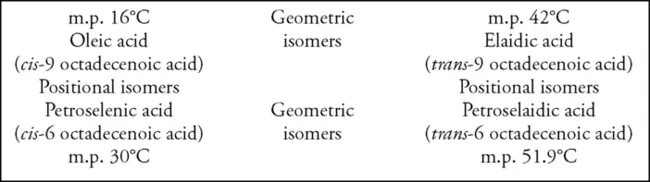
Oleic acid is the most common unsaturated fatty acid found in plant and animal fats. Since it is not especially vulnerable to oxidation, oils in which it is the predominant unsaturated fatty acid, such as olive oil and groundnut oil, have good flavor stability. Palmitoleic aci...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface to the Second Edition
- Preface to the First Edition
- Acknowledgments
- A Note on In-text Reference Citations in the 2009 Edition
- Chapter 1: Basic Components and Procedures
- Chapter 2: Adsorption
- Chapter 3: Adsorbents
- Chapter 4: Bleaching of Important Fats and Oils
- Chapter 5: Bleachers
- Chapter 6: Filtration and Filters
- Chapter 7: Oil Recovery
- Chapter 8: Safety, Security, and the Prevention of Error
- Chapter 9: Important Tests Relating to Bleaching
- Chapter 10: The Freundlich Isotherm in Studying Adsorption in Oil Processing
- Chapter 11: Enzymatic Degumming of Edible Oils and Fats
- References
- Index