
- 430 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Basic 1H- and 13C-NMR Spectroscopy
About this book
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful and theoretically complex analytical tool. Basic 1H- and 13C-NMR Spectroscopy provides an introduction to the principles and applications of NMR spectroscopy. Whilst looking at the problems students encounter when using NMR spectroscopy, the author avoids the complicated mathematics that are applied within the field. Providing a rational description of the NMR phenomenon, this book is easy to read and is suitable for the undergraduate and graduate student in chemistry.
- Describes the fundamental principles of the pulse NMR experiment and 2D NMR spectra
- Easy to read and written with the undergraduate and graduate chemistry student in mind
- Provides a rational description of NMR spectroscopy without complicated mathematics
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction
Publisher Summary
This chapter introduces and elucidates the structured and NMR as applied to organic compounds. The spectral analysis is used to identify structural features of these compounds. The advent of spectral analysis has allowed chemists to confirm whether a reaction has resulted in the desired product or not. Presently, several analytical techniques are available and depending on the area of study (organic, inorganic, physical chemistry, or biochemistry), the analytical methods may vary and the most commonly applied methods in organic chemistry are given for the reader. The IR spectroscopy provides information about the functional groups present in a molecule such as COR, COOR, CN, NO2, etc. The use of UV spectroscopy and mass spectrometry as applied in the study of structure of compound are briefly explained. Similarly, the mass spectroscopy provides information regarding molecular weight, or the formula weight of the compound and the X-ray spectroscopy is an excellent method to determine the structure of a compound. The NMR spectroscopy technique is a useful technique for identifying and analyzing organic compounds. The explanation of NMR spectroscopy is explained.
1.1 STRUCTURE ELUCIDATION AND NMR
Today several million organic compounds are known, and thousands of new ones are prepared each year. Industry and academia account for more than 90% of the organic compounds synthesized in the laboratory, while the rest are derived from natural resources. Spectral analysis is used to identify structural features of these compounds. The advent of spectral analysis has allowed chemists to confirm whether a reaction has resulted in the desired product or not. Presently, several analytical techniques are available. Depending on the area of study (organic, inorganic, physical chemistry, or biochemistry), the analytical methods may vary. The most commonly applied methods in organic chemistry are as follows:
(1) infrared (IR) spectroscopy;
(2) ultraviolet (UV) spectroscopy;
(3) mass spectrometry;
(4) X-ray spectroscopy;
(5) nuclear magnetic resonance (NMR) spectroscopy;
(6) elemental analysis.
Now let us focus on the kind of molecular structure information that can be obtained using these methods. The compound ethyl benzoate (1) will be used as an example to illustrate these methods.
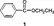
1.1.1 IR spectroscopy
IR spectroscopy provides information about the functional groups present in a molecule such as COR, COOR, CN, NO2, etc. The IR spectrum of ethyl benzoate will reveal the existence of an ester and double bond. This method of spectroscopy does not provide information regarding the location or connectivity of these functional groups.
1.1.2 UV spectroscopy
The principal use of UV spectroscopy is to identify structures containing conjugation. The existence of a benzene ring in conjugation with another system is evident from the UV spectrum of ethyl benzoate. In some instances, it is difficult to interpret this spectrum completely by UV and IR spectroscopy.
1.1.3 Mass spectrometry
Mass spectrometry provides information regarding molecular weight, or the formula weight of a compound. In the case of ethyl benzoate, analysis reveals the formula weight of 150 amu. Also, high-resolution mass spectroscopy (HRMS) may be used to determine the empirical formula of the molecule. In the case of ethyl benzoate, the empirical formula is C9H10O2. However, there may be hundreds of constitutional isomers of the same empirical formulae, making it difficult to distinguish these isomers. Even though the ethyl benzoate can be detected amongst these isomers, it is almost impossible to do so for molecules having a more complex structure.
1.1.4 X-ray spectroscopy
X-ray spectroscopy is an excellent method to determine the structure of a compound. However, this technique requires the availability of a compound as a single crystal. Most chemists find this process very tedious, time consuming and it requires a skillful hand. In the event when other spectral methods fail to reveal a compound’s identity, X-ray spectroscopy is the method of choice for structural determination where the other parameters such as bond lengths and bond angles are also determined. However, advances in technology have made it possible to have an NMR spectrum complete in as little as one minute.
1.1.5 NMR spectroscopy
NMR spectroscopy is a useful technique for identifying and analyzing organic compounds. This extremely important experimental technique is based on magnetic nuclear spin of 1H, 13C, 15N, 19F, 31P, and so forth. Only 1H and 13C will be considered in this example. Proton and carbon NMR spectra of ethyl benzoate (Figure 1) contain more detailed and definitive information:
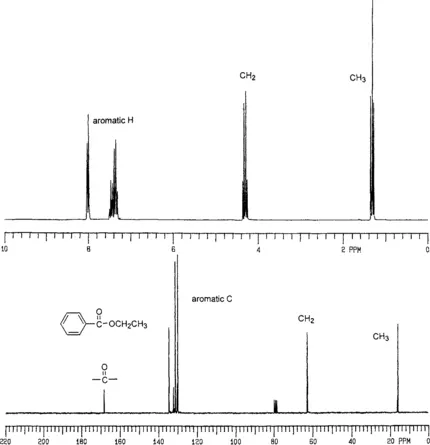
(1) 1H-NMR provides information about the number of protons in the molecule.
(2) 1H-NMR reveals the existence of an aromatic moiety that is monosubstituted. This is indicative of 5 protons attached to an aromatic ring.
(3) The existence of a methyl group and its attachment to a CH2 group is indicated.
(4) Analysis of the signals provides information about a CH3 group being adjacent to a methylene group that is attached to electronegative atom (oxygen).
(5) 13C NMR provides information about the number of carbons in the molecule.
(6) The existence of a carbonyl carbon and four different carbon atoms on an aromatic ring (monosubstituted benzene ring) is revealed from the 13C NMR.
The combination of all analytical information shows that the molecule is indeed ethyl benzoate. Hence, when information obtained from NMR and other analytical methods is compared, it is apparent that the most detailed and necessary information to recognize a structure is obtained from NMR spectra. However, this does not mean that NMR is always sufficient for structural analysis. For absolute analysis of structures, it is necessary to apply all spectroscopic methods. If a new compound is synthesized, the chemist who does scientific research should have all the spectroscopic data related to this chemical compound. The information provided by an NMR spectrum is useful only if it can be interpreted well.
In addition to providing information related to constitutional analysis, NMR spectra provide detailed information pertinent to configurational and conformational analysis. Illustrations of the use of NMR in distinguishing these aspects will be explored. In the classical reaction of nitration of acetophenone (2), the nitro group attaches to the meta position.

Conventionally, it is accepted that nitration occurs at the meta position, but how can this be proved? There must be a method to determine the position of the nitro group (the constitutional analysis of the molecule). Indeed, this question is easily answered by the use of NMR.
Most chemical reactions produce configurational isomers. For instance, there may be two products (5 and ...
Table of contents
- Cover image
- Title page
- Table of Contents
- Preface
- Chapter 1: Introduction
- Chapter 2: Resonance Phenomena
- Chapter 3: Chemical Shift
- Chapter 4: Spin–Spin Splitting in 1H-NMR Spectra
- Chapter 5: Spin–Spin Splitting to Different Protons
- Chapter 6: Spin Systems: Analysis of the 1H-NMR Spectra
- Chapter 7: NMR Shift Reagents and Double Resonance Experiments: Simplification of the NMR Spectra
- Chapter 8: Dynamic NMR Spectroscopy
- Chapter 9: Introduction
- Chapter 10: Absorption and Resonance
- Chapter 11: Pulse NMR Spectroscopy
- Chapter 12: Chemical Shift
- Chapter 13: 13C Chemical Shifts of Organic Compounds
- Chapter 14: Spin–Spin Coupling
- Chapter 15: Multiple-Pulse NMR Experiments
- Chapter 16: Two-Dimensional (2D) NMR Spectroscopy
- Solutions to Exercises
- References
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Basic 1H- and 13C-NMR Spectroscopy by Metin Balci in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.