1 Background
Of the 94 naturally occurring elements, 70 are metals, broadly defined as elements which are good conductors of electricity and heat, which form cations by loss of electrons, and which yield basic oxides and hydroxides. A few others, including Se and As, are honorary metals (“metalloids”), sharing some but not all properties of true metals. The terms “heavy metal” (universally viewed in a negative light by the general public) and “light metal” (often positively viewed) are outdated and chemically meaningless (Duffus, 2002; Hodson, 2004). Various other classifications have been proposed, of which the most scientifically defensible appears to be that based on their Lewis acid behavior (Lewis, 1923), as articulated by Nieboer and Richardson (1980). In this scheme, “hard” class A metals (e.g. Na, Mg, K, Ca, Rb, Li, U, Al) tend to bond ionically with oxygen donors, while “soft” class B metals (e.g. Ag, Hg, Pb, Cu) tend to bond covalently with sulfur donors. Unfortunately, this classification has proven unpopular with aquatic toxicologists, perhaps because so many important metals (e.g. Co, Cd, Ni, Cr, Fe, Zn) fall between the cracks as borderline or intermediate class metals, and their classification is controversial.
Metals have been long prized by humans for their generally attractive appearance (lustrous and shiny), malleability when heated, and hardness when cold, especially when blended in alloys, which gives them great practical utility for the making of tools, machines, weapons, and structures. The computer on which this manuscript was typed contains 30–40 different metals. The exploitation of metals by humans goes back to at least 6000 BC (the end of the Neolithic period or perhaps even earlier), but amazingly, up until the end of the Dark Ages (around AD 1400), only seven metals had been firmly identified and were in common use: Au, Cu, Ag, Pb, Sn, Hg, and Fe. With respect to the latter, Pliny the Elder wrote, “the ores of iron provide a metal which is at once the best servant of mankind–but the blame for death must be credited to man and not to nature”. Many of the metals that we take for granted today (e.g. Co, Mn, Mo, Zn, Cd, Ni, Cr, Al) were only discovered in the eighteenth and nineteenth centuries. Indeed, only in the twentieth century was it realized that many of these same metals (Cu, Fe, Mn, Mo, Zn, Cr, and Co, plus probably Ni, Ge, Rb, and V in some organisms) are “essential”, i.e. absolutely required in trace amounts for biological life owing to their participation in metabolic reactions as cofactors or integral parts of enzymes (Jeffery, 2001). There are no known biological functions for “non-essential” metals, which means that physiological mechanisms for specifically taking up such metals into an organism theoretically should not have evolved.
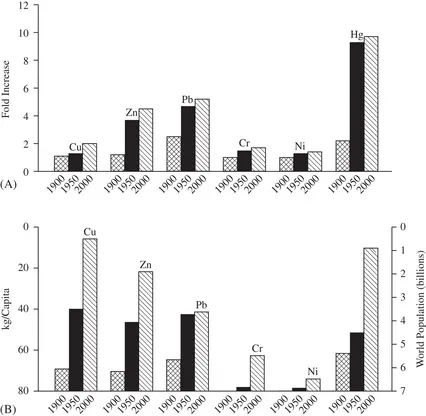
As elements, metals can be neither created nor destroyed, so once they are extracted from ores, they are ultimately dispersed into the environment. The vast majority of this extraction and dispersion (“production”) has occurred since 1900, with production rates increasing in a quasi-exponential fashion throughout the last century. On a global basis, anthropogenically driven metal fluxes through the environment account for approximately half of all metal fluxes, and most metals are being “produced” at a rate that is orders of magnitude higher than the natural rate of renewal in the Earth’s crust (i.e. by molten core upwelling and meteorite deposition) (Rauch and Graedel, 2007; Rauch and Pacyna, 2009). For a range of commonly used metals, cumulative world production by the year 2000 had reached levels many times higher than estimated levels in the year 1900: for example, Cr (643×), Ni (110×), Cu (25×), Zn (22×), Cd (18×), and Hg (7.3×) (Han et al., 2002). One notable exception is Pb (only 2–3×), a metal that was heavily exploited in “preindustrial” times, and whose production in the latter part of the twentieth century was greatly curtailed owing to health concerns and efficient recycling. If dispersed homogeneously throughout the world’s soils and sediments, this cumulative anthropogenic production would have increased the levels of most metals to two- to ten-fold above natural background, as illustrated in Fig. 1.1(A). Note that the largest increases are for Hg, mainly owing to prolific burning of coal in which it is a trace contaminant. The smallest increases are for Cr and Ni, for which exploitation only started in about 1950. Of course, dispersion is a slow, non-homogeneous process, so while there are regions where natural background concentrations still persist, yet other areas have metal levels that are orders of magnitude higher, owing to local anthropogenic contamination. Dividing estimated cumulative production by population is another way of putting these data into perspective; note the large increases in the cumulative metal burden per capita, despite the greater than four-fold increase in world population from 1900 to 2000 (Fig. 1.1B).
While there were some important early studies in the aquatic toxicology of metals (e.g. Jones, 1939; Holm-Jensen, 1948), prior to about 1950, there was general belief in “better living through chemistry”, and relatively little public and scientific concern about the dispersion of metals in the environment or their toxicological effects. The growth of such concern paralleled the growth of the environmental movement, catalyzed by the publication of Silent Spring by Rachel Carson (1962). This landmark book focused mainly on organic pollutants, especially pesticides and herbicides, rather than on metals. Nevertheless, it fundamentally shifted the landscape towards environmental awareness for all potential pollutants. The establishment of national environmental protection agencies [e.g. US Environmental Protection Agency (EPA) in 1970, Environment Canada in 1971] in many jurisdictions ensued in the following two decades, together with efforts to establish national water quality guidelines for various contaminants, including metals. Simultaneously, there was a massive surge in aquatic toxicological research, which has provided the data critical for developing water guidelines and criteria for metals, many of which remain in use today.
Some remarkable studies in fish toxicology from this era blurred the traditional boundaries with both physiology and geochemistry, by addressing mechanisms of toxicity and showing that the impacts of metals depended on what else was present in the water (Lloyd and Herbert, 1962; Brown, 1968; Zitko et al., 1973; Brown et al., 1974; Pagenkopf et al., 1974; Zitko and Carson, 1976; Chakoumakos et al., 1979). The interests of physiologists and geochemists were thereby piqued. The international journals Aquatic Toxicology and Environmental Toxicology and Chemistry were founded just 30 years ago, in 1981 and 1982, respectively. Another important driving force was the acid rain crisis of the 1970s and 1980s, when focused research revealed that many of the effects originally attributed to the acidity of the water alone were in fact due to metals which became dissolved and/or more toxic at low pH (see Couture and Pyle, Chapter 9). This was particularly true of Al (see Wilson, Chapter 2, Vol. 31B). There followed a surge of mechanistic research which continues to this day, and which forms the basis for these two volumes. Much of this research has been sponsored by government agencies and various metal-producing industries, often in cooperation, because of common interests in regulatory issues. In this regard, the European Union (EU), the USA, Canada, Australia/New Zealand, China, and several Latin American countries have recently revised, or are in the process of revising, their ambient water quality criteria (AWQC) for metals, making the present volumes timely.
Since about 1990, there has been an explosion of new information on the molecular, cellular, and organismal handling of metals in fish. Much of this research has focused on the physiological mechanisms of metal uptake, toxicity, and excretion. Internally, homeostatic mechanisms have been characterized which include regulated storage and detoxification (e.g. metallothioneins, ferritin, glutathione) and vehicles for transporting metals around the body in the circulation (e.g. ceruloplasmin, transferrin). New molecular, genomic, and proteomic techniques are now facilitating precise characterization of these pathways, and how they respond to environmental challenges. All these topics are major themes in the various metal-specific chapters. In turn, this information has proven useful in interpreting the responses of fish populations in the wild to chronic metal contamination (see Couture and Pyle, Chapter 9). Very importantly, new physiological and geochemical information has now been...