
This is a test
- 248 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Book details
Book preview
Table of contents
Citations
About This Book
Fusion: The Energy of the Universe, 2e is an essential reference providing basic principles of fusion energy from its history to the issues and realities progressing from the present day energy crisis. The book provides detailed developments and applications for researchers entering the field of fusion energy research. This second edition includes the latest results from the National Ignition Facility at the Lawrence Radiation Laboratory at Livermore, CA, and the progress on the International Thermonuclear Experimental Reactor (ITER) tokamak programme at Caderache, France.
- Comprehensive coverage— basic principles, detailed developments and practical applications
- Wide accessibility, but with sufficient detail to keep the technical reader engaged
- Details the initial discovery of nuclear fusion, current attempts to create nuclear fusion here on earth and today's concern over future energy supply
- Color illustrations and examples
- Includes technical notes for aspiring physicists
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Fusion by Garry McCracken,Peter Stott in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Optics & Light. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
What Is Nuclear Fusion?
1.1 The Alchemists’ Dream
In the Middle Ages, the alchemists’ dream was to turn lead into gold. The only means of tackling this problem were essentially chemical ones, and these were doomed to failure. During the 19th century, the science of chemistry made enormous advances, and it became clear that lead and gold are different elements that cannot be changed into each other by chemical processes. However, the discovery of radioactivity at the very end of the 19th century led to the realization that sometimes elements do change spontaneously (or transmute) into other elements. Later, scientists discovered how to use high-energy particles, either from radioactive sources or accelerated in the powerful new tools of physics that were developed in the 20th century, to induce artificial nuclear transmutation in a wide range of elements. In particular, it became possible to split atoms (the process known as nuclear fission) or to combine them (the process known as nuclear fusion). The alchemists (Figure 1.1) did not understand that their quest was impossible with the tools they had at their disposal, but in one sense it could be said that they were the first people to search for nuclear transmutation.

Figure 1.1 An alchemist in search of the secret that would change lead into gold. Because alchemists had only chemical processes available, they had no hope of making the nuclear transformation required. From a painting by David Teniers the younger, 1610–1690.
What the alchemists did not realize was that nuclear transmutation was occurring before their very eyes, in the Sun and in all the stars of their night sky. The processes in the Sun and stars, especially the energy source that had sustained their enormous output for eons, had long baffled scientists. Only in the early 20th century was it realized that nuclear fusion is the energy source that runs the universe and that simultaneously it is the mechanism responsible for creating all the different chemical elements around us.
1.2 The Sun’s Energy
The realization that the energy radiated by the Sun and stars is due to nuclear fusion followed three main steps in the development of science. The first was Albert Einstein’s famous deduction in 1905 that mass can be converted into energy. The second step came a little over 10 years later, with Francis Aston’s precision measurements of atomic masses, which showed that the total mass of four hydrogen atoms is slightly larger than the mass of one helium atom. These two key results led Arthur Eddington and others, around 1920, to propose that mass could be turned into energy in the Sun and the stars if four hydrogen atoms combine to form a single helium atom. The only serious problem with this model was that, according to classical physics, the Sun was not hot enough for nuclear fusion to take place. It was only after quantum mechanics was developed in the late 1920s that a complete understanding of the physics of nuclear fusion became possible.
Having answered the question as to where the energy of the universe comes from, physicists started to ask how the different atoms arose. Again fusion was the answer. The fusion of hydrogen to form helium is just the start of a long and complex chain. It was later shown that three helium atoms can combine to form a carbon atom and that all the heavier elements are formed in a series of more and more complicated reactions. Nuclear physicists played a key role in reaching these conclusions. By studying the different nuclear reactions in laboratory accelerators, they were able to deduce the most probable reactions under different conditions. By relating these data to the astrophysicists’ models of the stars, a consistent picture of the life cycles of the stars was built up and the processes that give rise to all the different atoms in the universe were discovered.
1.3 Can We Use Fusion Energy?
When fusion was identified as the energy source of the Sun and the stars, it was natural to ask whether the process of turning mass into energy could be demonstrated on Earth and, if so, whether it could be put to use for man’s benefit. Ernest Rutherford, the famous physicist and discoverer of the structure of the atom, made this infamous statement to the British Association for the Advancement of Science in 1933: “We cannot control atomic energy to an extent that would be of any use commercially, and I believe we are not ever likely to do so.” It was one of the few times when his judgment proved wanting. Not everybody shared Rutherford’s view; H. G. Wells had predicted the use of nuclear energy in a novel published in 1914.1
The possibility of turning nuclear mass into energy became very much more real in 1939, when Otto Hahn and Fritz Strassman demonstrated that the uranium atom could be split by bombarding uranium with neutrons, with the release of a large amount of energy. This was fission. The story of the development of the fission chain reaction, fission reactors, and the atom bomb has been recounted many times. The development of the hydrogen bomb and the quest for fusion energy proved to be more difficult. There is a good reason for this. The uranium atom splits when bombarded with neutrons. Neutrons, so called because they have no electric charge, can easily penetrate the core of a uranium atom, causing it to become unstable and to split. For fusion to occur, two hydrogen atoms have to get so close to each other that their cores can merge; but these cores carry strong electric charges that hold them apart. The atoms have to be hurled together with sufficiently high energy to make them fuse.
1.4 Man-Made Suns
The fusion reaction was well understood by scientists making the first atomic (fission) bomb in the Manhattan Project. However, although the possibility that fusion could be developed as a source of energy was undoubtedly discussed, no practical plans were put forward. Despite the obvious technical difficulties, the idea of exploiting fusion energy in a controlled manner was seriously considered shortly after World War II, and research was started in the UK at Liverpool, Oxford, and London universities. One of the principal proponents was George Thomson, the Nobel Prize-winning physicist and son of J. J. Thomson, the discoverer of the electron. The general approach was to try to heat hydrogen gas to a high temperature so that the colliding atoms have sufficient energy to fuse together. By using a magnetic field to confine the hot fuel, it was thought that it should be possible to allow adequate time for the fusion reactions to occur. Fusion research was taken up in the UK, the US, and the Soviet Union under secret programs in the 1950s and subsequently, after being declassified in 1958, in many of the technically advanced countries of the world. The most promising reaction is that between the two rare forms of hydrogen, called deuterium and tritium. Deuterium is present naturally in water and is therefore readily available. Tritium is not available naturally and has to be produced in situ in the power plant. This can be done by using the products of the fusion reaction to interact with the light metal lithium in a layer surrounding the reaction chamber in a breeding cycle. Thus the basic fuels for nuclear fusion are lithium and water, both readily and widely available. Most of the energy is released as heat that can be extracted and used to make steam and drive turbines, as in any conventional power plant. A schematic diagram of the proposed arrangement is shown in Figure 1.2. The problem of heating and containing the hot fuel with magnetic fields (magnetic-confinement fusion) turned out to be much more difficult than at first envisaged.
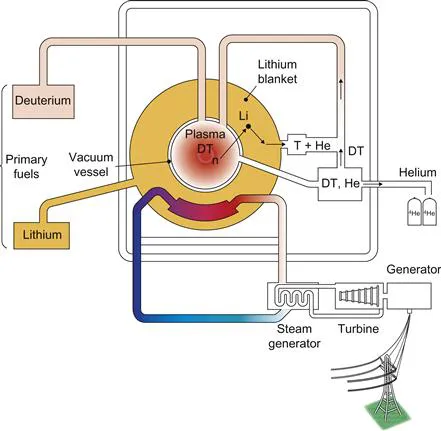
Figure 1.2 Schematic diagram of a proposed nuclear fusion power plant. The deuterium and tritium fuel burns at a very high temperature in the central reaction chamber. The energy is released as charged particles, neutrons, X-rays, and ultraviolet radiation and it is absorbed in a lithium blanket surrounding the reaction chamber. The neutrons convert the lithium into tritium fuel. A conventional steam-generating plant is used to convert the nuclear energy to electricity. The waste product from the nuclear reaction is helium.
However, research on the peaceful use of fusion energy was overtaken in a dramatic way with the explosion of the hydrogen bomb in 1952. This stimulated a second approach to controlled fusion, based on the concept of heating the fuel to a sufficiently high temperature very quickly before it has time to escape. The invention of the laser in 1960 provided a possible way to do this; lasers can focus intense bursts of energy onto small targets. The idea is to rapidly heat and compress small fuel pellets or capsules in a series of mini-explosions. This is called inertial confinement because the fusion fuel is confined only by its own inertia. Initially, the expertise was limited to those countries that already had nuclear weapons, and some details still remain secret, although other countries have now taken it up for purely peaceful purposes. Apart from the heating and confinement of the fuel, the method of converting fusion energy into electricity will be very similar to that envisaged for magnetic confinement.
1.5 The Rest of the Story
The considerable scientific and technical difficulties encountered by the magnetic and inertial-confinement approaches have caused these programs to stretch over many years. The quest for fusion has proved to be one of the most difficult challenges faced by scientists. After many years, the scientific feasibility of thermonuclear fusion via the magnetic-confinement route has been demonstrated, and inertial-confinement experiments are expected to reach a similar position soon. Developing the technology and translating these scientific achievements into power plants that are economically viable will be a major step that will require much additional time and effort. Some have hoped that they could find easy ways to the rewards offered by fusion energy. This line of thinking has led to many blind alleys and even to several false claims of success, the most widely publicized being the so-called “cold fusion” discoveries that are described in Chapter 8.
1. Atomic energy and nuclear energy are the same thing.
Chapter 2
Energy from Mass
2.1 Einstein’s Theory
Energy is something with which everyone is familiar. It appears in many different forms, including electricity, light, heat, chemical energy, and motional (or kinetic) energy. An important scientific discovery in the 19th century was that energy is conserved. This means that energy can be converted from one form to another, but the total amount of energy must stay the same. Mass is also very familiar, though sometimes it is referred to, rather inaccurately, as weight. On the Earth’s surface, mass and weight are often thought of as being the same thing, and they do use the same units—something that weighs 1 kilogram has a mass of 1 kilogram—but strictly speaking weight is the force that a mass experiences in the Earth’s gravity. An object always has the same mass, even though in outer space it might appear to be weightless. Mass, like energy, is conserved.
The extraordinary idea that mass and energy are equivalent was proposed by Albert Einstein (Figure 2.1) in a brief three-page paper published in 1905. At that time, Einstein was a young man who was virtually unknown in the scientific world. His paper on the equivalence of mass and energy was followed soon after by three seminal papers—on the photoelectric effect, on Brownian motion, and on special relativity—all published in the same year. Henri Becquerel had discovered radioactivity 10 years previously. Using simple equations and application of the laws of conservation of energy and momentum, Einstein argued that the atom left after a radioactive decay event had emitted energy in the form of radiation must be less massive than the original atom. From this analysis he deduced that “If a body gives off the energy E in the form of radiation, its mass diminishes by E/c2.” He went on to say, “It is not impossible that with bodies whose energy content is variable to a high degree (e.g., radium salts) the theory may be successfully put to the test.”

Figure 2.1 Photograph of Albert Einstein in 1921—the year in which he received the Nobel Prize in Physics. Einstein (1879–1955) had graduated in 1901 and had made a number of applications for academic jobs, without success. He eventually got a job as technical expert, third class, in the Swiss patent office in Berne, which meant that he had to do all his research in his spare time.
Einstein’s deduction is more commonly written as E=mc2, probably the most famous equation in physics. It states that mass is another form of energy and that energy equals mass multiplied by the velocity of light squared. Although it took a long time to get experimental proof of this entirely theoretical prediction, we now know that it was one of the most significant advances ever made in science.
2.2 Building Blocks
To see how Einstein’s theory led to the concept of fusion energy, we need to go back to the middle of the 19th century. As the science of chemistry developed, it became clear that everything is built up from a relatively small number of basic components, called elements. At that time, about 50 elements had been identified, but we now know that there are around 1...
Table of contents
- Cover Image
- Table of Contents
- Title
- Copyright
- Dedication
- Technical Summaries
- Foreword to the Second Edition
- Foreword to the First Edition
- Preface
- Acknowledgments
- Chapter 1. What Is Nuclear Fusion?
- Chapter 2. Energy from Mass
- Chapter 3. Fusion in the Sun and Stars
- Chapter 4. Man-Made Fusion
- Chapter 5. Magnetic Confinement
- Chapter 6. The Hydrogen Bomb
- Chapter 7. Inertial-Confinement Fusion
- Chapter 8. False Trails
- Chapter 9. Tokamaks
- Chapter 10. From T3 to ITER
- Chapter 11. ITER
- Chapter 12. Large Inertial-Confinement Systems
- Chapter 13. Fusion Power Plants
- Chapter 14. Why We Will Need Fusion Energy
- Units
- Glossary
- Further Reading
- Index