![]()
Section X
Brain Peptides
Chapter 96 Apelin
Chapter 97 BNP/CNP
Chapter 98 CART
Chapter 99 CCK
Chapter 100 CGRP/Adrenomedullin
Chapter 101 CRH Family
Chapter 102 Endozepines
Chapter 103 Galanin and GALP
Chapter 104 Ghrelin
Chapter 105 GHRH
Chapter 106 GnRH (LHRH)
Chapter 107 Gonadotropin-Inhibitory Hormone
Chapter 108 Hypocretins (Orexins)
Chapter 109 Kisspeptins
Chapter 110 MCH
Chapter 111 Melanocortins
Chapter 112 MIF-1/Tyr-MIF-1
Chapter 113 Nesfatin-1
Chapter 114 Neuromedin U
Chapter 115 Neuropeptide B/W
Chapter 116 Neuropeptide S
Chapter 117 Neurotensin/Neuromedin N
Chapter 118 NPY
Chapter 119 PACAP
Chapter 120 Prolactin-Releasing Peptide
Chapter 121 Relaxins
Chapter 122 26RFa
Chapter 123 Secretin
Chapter 124 Somatostatin/Cortistatin
Chapter 125 Tachykinins
Chapter 126 TRH
Chapter 127 Urotensin II Peptides
Chapter 128 VIP
Chapter 129 VP/OT
![]()
Chapter 96
Apelin
Xavier Iturrioz and Catherine Llorens-Cortes
ABSTRACT
The APJ receptor was originally isolated as an orphan seven-transmembrane-domain G-protein-coupled receptor in humans. Its endogenous ligand, apelin, was identified in 1998 and is generated from a 77-amino-acid precursor, preproapelin. Apelin and its receptor are expressed in hypothalamic vasopressinergic and oxytocinergic neurons. Apelin acts as a potent aquaretic neuropeptide, counteracting vasopressin actions. Apelin may also act on the kidney to increase diuresis. Central injection of apelin in lactating rats inhibits hypothalamic parvocellular and magnocellular OXY neuron activity in a direct autocrine or paracrine manner, reducing the amount of milk ejected for the pups. Apelin exerts peripheral actions, decreasing mean arterial blood pressure and improving myocardium contractility and load. Altogether these results suggest that apelin may be a key peptide for the fine regulation of functions relating to the maintenance of body fluid homeostasis in certain physiological conditions and plays a crucial role in the regulation of cardiovascular functions.
Discovery
The apelin story began in 1993 with the cloning of the cDNA of the orphan APJ receptor (putative receptor protein related to the type 1 angiotensin receptor) from a human genomic library, which was subsequently cloned in rodents.6,23,24 The human receptor is 380 amino acids long and is a member of the family of orphan seven-transmembrane-domain G-protein-coupled receptors (GPCRs). Its amino acid sequence was found to be 31% identical to that of the human type 1 angiotensin (AT1) receptor, but it does not bind radiolabeled angiotensin II (Ang II), and exposure of the rat APJ receptor to Ang II does not modify cyclic adenosine monophosphate (cAMP) production, showing that this receptor is not an angiotensin receptor subtype. It therefore remained an orphan GPCR for which the endogenous ligand had to be isolated. The APJ receptor was deorphanized in 1998 when apelin, its endogenous ligand, was isolated from bovine stomach tissue extracts.34 This ligand was named “apelin” for the endogenous ligand for the APJ receptor.
Structure of the mRNA/Gene
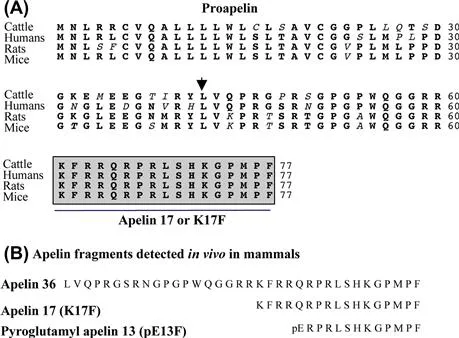
Apelin is a 36-amino-acid peptide (apelin-36) generated from a larger precursor, the 77-amino-acid proapelin (Fig. 1A). The human preproapelin gene is located on chromosome X at locus Xq25–q26.1 and contains three exons, with the coding region spanning exons 1 and 2. The 3′-untranslated region also spans two exons (2 and 3). This may account for the presence of transcripts of two different sizes (~3 and ~3.6 kb) in various tissues.23
FIGURE 1 (A) Amino acid sequences of the apelin precursor, preproapelin, in cattle, human, rat, and mouse. The first amino acid of apelin-36 is indicated by an arrowhead and the apelin-17 sequence is indicated by a box.34 (B) Apelin fragments detected in vivo in mammals: apelin-36, apelin-17 (K17F), and the pyroglutamyl form of apelin-13 (pE13F).
Processing of the Precursor
The alignment of preproapelin amino acid sequences from cattle, human, rat, and mouse shows strict conservation of the C-terminal 17 amino acids, known as apelin-17 or K17F (Fig. 1B). In vivo, proapelin gives rise to various molecular forms of apelin of 36, 17, and 13 amino acids, but the mechanism implicated in this processing remains unknown. The presence of pairs of basic residues in proapelin suggests that prohormone convertases could be implicated in this processing. In the brain and plasma of rat, the predominant forms of apelin are the pyroglutamyl forms of apelin-13 (pE13F) and, to a lesser extent, K17F (Fig. 1B).7 In rat lung, testis, and uterus and bovine colostrum, apelin-36 predominates, whereas, in the rat mammary gland, both apelin-36 and pE13F are present.10,15 In human heart, pE13F has been identified as the predominant molecular form of apelin,17 whereas in human plasma, K17F predominates followed by pE13F and to a lesser extent, apelin-36.1
Distribution of the mRNA/Peptide
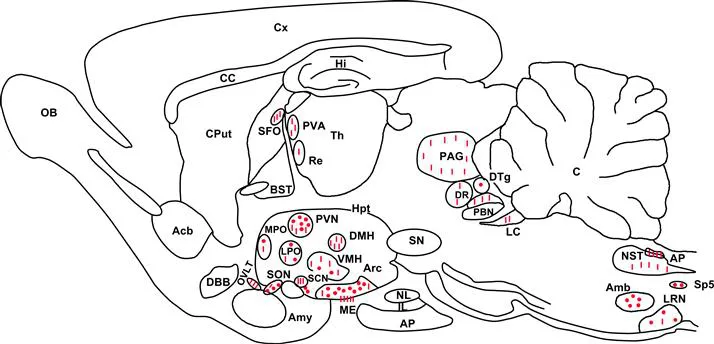
The apelin precursor, mRNA is expressed in the brain and also in the lung, heart, kidney, adipose tissue, and vascular endothelium.3,14,16,21,26,27 The development of a polyclonal antibody with high affinity and selectivity for K17F has made it possible to visualize, for the first time, apelin-containing neurons in the central nervous system of rats.26 The precise neuroanatomical distribution of apelin immunoreactivity shows that apelin-immunoreactive (IR) cell bodies are particularly abundant in the structures of the hypothalamus and medulla oblongata involved in the control of neuroendocrine activities, drinking behavior, and arterial blood pressure (BP), notably in the hypothalamic supraoptic nucleus (SON) and the magnocellular part of the paraventricular nucleus (PVN), the arcuate nucleus, the lateral reticular nucleus, and the nucleus ambiguus (Fig. 2).26,27 Conversely, apelin-IR nerve fibers are much more widely distributed in many regions of the brain. The density of IR nerve fibers and apelinergic nerve endings is the highest in the inner layer of the median eminence and in the posterior pituitary,4,27 suggesting that apelin neurons of the SON and PVN, like magnocellular AVP and oxytocin neurons, project into the posterior pituitary. In support of this notion, double immunofluorescence staining showed that apelin colocalizes with AVP7,29 and oxytocin2,4 in magnocellular hypothalamic neurons in rats.
FIGURE 2 Neuroanatomical distribution of apelin-IR cell bodies and nerve fibers on a parasagittal section of adult colchicine-treated rat brain. Apelin-IR cell bodies and nerve fibers are shown as dots and lines, respectively.
Abbreviations: Acb, nucleus accumbens; Amb, nucleus ambiguus; Amy, amygdala; AP, anterior pituitary; AP: area postrema; Arc, arcuate nucleus of the hypothalamus; BST, bed nucleus of the stria terminalis; C, cerebellum; CC, corpus callosum; Cput, caudate putamen; Cx, cerebral cortex; DBB, diagonal band of Broca; DMH, dorsomedial nucleus of the hypothalamus; DR, dorsal raphe nucleus; DTg, dorsal tegmental nucleus; Hi, hippocampus; Hpt, hypothalamus; IL, intermediate lobe of the pituitary; LC, locus coeruleus; LPO: lateral preoptic area; LRN, lateral reticular nucleus; ME, median eminence; MPO, medial preoptic nucleus; NL, neural lobe of the pituitary; NST, nucleus of the solitary tract; OB, olfactory bulb; OVLT: vascular organ of the lamina terminalis; PAG, periaqueductal gray; PBN, parabrachial nucleus; PVA, paraventricular thalamic nucleus; PVN, paraventricular nucleus of the hypothalamus; Re, reuniens thalamic nucleus; SCN, suprachiasmatic nucleus; SFO, subfornical organ; SN, substantia nigra; SON, supraoptic nucleus; Sp5, spinal trigeminal nucleus; S, septum; Th, thalamus; VMH, ventromedial nucleus of the hypothalamus. Adapted from Reaux et al.27
Apelin-IR nerve fibers also innervate the median preoptic nucleus, mesencephalon, pons, medulla oblongata, and several circumventricular organs such as the vascular organ of the lamina terminalis (OVLT), the subfornical organ (SFO), the subcommissural organ, and the area postrema.27
Receptors and Signaling Cascades
Distribution of the Apelin Receptor
Like apelin, the apelin receptor mRNA is widely distributed throughout the central nervous system6,16,21,23 of rats and is also present in peripheral tissues such as heart, vascular endothelium, kidney, adipose tissue, spleen, muscle, and uterus.8,10,11,14,21 In situ hybridization analysis of apelin receptor mRNA expression in the adult rat brain shows intense labeling in the hypothalamus, especially in the SON and PVN and to a lesser extent in the arcuate nucleus. The anterior and intermediate lobes of the pituitary are also strongly labeled, as well as the pineal gland. Labeling is also found in extrahypothalamic structures such as the piriform cortex, the nucleus of the lateral olfactory tract, the central gray matter, the structures containing monoaminergic neuronal cell bodies (pars compacta of the substantia nigra, dorsal raphe nucleus, and locus coeruleus), the entorhinal cortex, the dentate gyrus and Ammon’s horn.6 Further, double-labeling studies combining immunocytochemistry and in situ hybridization in rats have shown that in SON and PVN apelin receptors,23,26 like type 1A and 1B AVP receptors (V1A and V1B), are synthesized by magnocellular AVP neurons suggesting an interaction between AVP and apelin. More recently, Bodineau et al. have shown that apelin receptor mRNA is expressed by magnocellular and parvocellular oxytocin neurons in the SON and PVN, suggesting an interaction between oxytocin and apelin.2
Signaling Cascades
The human and rat apelin receptors are 380 and 377 amino acids long, respectively, and belong to class A seven-transmembrane GPCRs. These receptors share high identity (90%) in amino acid sequence. Rat and human apelin receptors are negatively coupled to adenylyl cyclase.6,21 Indeed, apelin-36, K17F, apelin-13 (Q13F), and pE13F are potent inhibitors of forskolin-induced cAMP production in CHO or HEK 293 cells stably expressing the rat or human receptor.6,21 Masri et al. have shown that both apelin-36 and pE13F activate Gαi1 and Gαi2 isoforms but not Gαi3 in CHO cells stably expressing the murine apelin receptor.20 Ala-scan of pE13F21 and N- or C-terminal deletions of K17F 9 showed that the arginine residues in positions 2 and 4 or the leucine residue in position 5 in pE13F play a critical role in binding affinity and inhibition of cAMP production. Apelin-36, K17F, and pE13F also increase intracellular calcium mobilization in several cell lines such as NTera 2 human teratocarcinoma cells or HEK 293 and RBL-2H3 cells stably expressing the human apelin receptor.5,21 Several kinases are activated by apelin receptor stimulation in different cell types. Masri et al. have shown that pE13F activates extracellular signal-regulated kinases (ERKs) in CHO cells stably expressing the mouse apelin receptor, via a pertussis-toxin-sensitive G protein, a PKC-dependent pathway, and in a Ras-independent pathway.18 Apelin also activates p70S6 kinase in human umbilical vein endothelial cells (HUVEC) and in CHO cells expre...