
- 264 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Painless Chemistry
About this book
Barron's makes learning Chemistry fun and PAINLESS!
Learning at home is now the new normal. Need a quick and painless refresher? Barron’s Painless books make learning easier while you balance home and school.
Painless Chemistry provides lighthearted, step-by-step learning and includes:
Learning at home is now the new normal. Need a quick and painless refresher? Barron’s Painless books make learning easier while you balance home and school.
Painless Chemistry provides lighthearted, step-by-step learning and includes:
- Complex topics broken down with examples and illustrations, including atomic theory, chemical bonding, the structure of molecules, and more
- The Periodic Table of Elements and how it offers the key to understanding Chemistry
- Painless tips, instructive tables,“Brain Tickler” quizzes and answers throughout each chapter, and more.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Matter
Energy or Matter
Chemistry is the division of physical science that studies the composition, properties, and reactions of substances. The nature of matter and energy have been investigated and debated from ancient times.
Our universe is composed of energy and matter. Energy is the ability to do work. In chemical reactions, chemical energy is interconverted with other types of energy, such as light, heat, and electricity.
Matter has mass and inertia. Matter also takes up space (volume). The amount of matter in a specified space is density. For pure substances, density at a specific pressure and temperature is an identifying characteristic of the substance.
Mass
Mass is the amount of matter in an object. The basic unit of mass is the kilogram. One gram is the approximate mass of a 5-cm (2-inch) steel paper clip. A kilogram is 1,000 grams (2.2 pounds). One liter of water has a mass of 1 kilogram.
Common tools for measuring mass are the double pan balance, triple beam balance, and electronic balance. The double pan balance compares the mass of a substance to a known standard mass (a metric weight). The triple beam balance has an arm with sliding mass units that are adjusted to balance the mass of a substance. The electronic balance provides a digital measurement of the mass of a substance.
Mass versus weight
Weight is a measure of the pull or acceleration of gravity on an object’s mass (w = mg, or weight equals mass times the acceleration of gravity). The acceleration of gravity near Earth’s surface is 9.8 m/sec2. The weight of an object can change if gravity changes. The amount of matter in an object does not change even if the pull of gravity changes. Therefore, the mass of an object does not change even if the pull of gravity changes.
In many countries, the kilogram (kg) is commonly used to express weight. In science, kilogram is a metric unit of mass. The newton is a metric unit of weight, and is defined as the force needed to accelerate 1 kilogram of mass at a rate of 1 meter per second per second. One newton equals 1 kg · m/sec2. Another unit of force is the pound. One pound of force is about 4 newtons.
Sometimes in chemistry the term weight is used when mass is meant. Be careful to pay attention to units of measure.

Mass and Weight
Mass is the amount of matter in a substance.
The basic metric unit of mass is the kilogram.
The basic metric unit of mass is the kilogram.
Weight is the pull of gravity on an object.
The basic metric unit of weight is the newton.
The basic metric unit of weight is the newton.
On Earth 1 kilogram exerts a force of 9.8 newtons.
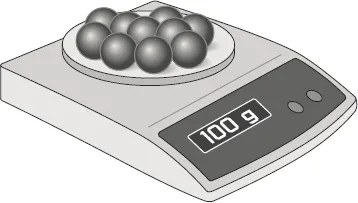
1.What is the total mass of the marbles shown in the illustration?
2.If each of the 10 glass marbles on the scale has the same mass, what is the average mass of a single marble? Show your calculation.
3.A brass plate has a mass of 2 kilograms on Earth. The gravity of the moon is one-sixth that of Earth’s gravity. What is the mass of the brass plate on the moon? Explain your answer.
(Answers are on page 16.)
Volume
Volume is the space that matter occupies or takes up. Volume is measured in three dimensions: length, width, and height. A common tool for measuring the volume of a regularly shaped solid is the metric ruler or meter stick. Common units of measure for solids are cubic centimeters (cm3) and cubic meters (m3).
A common tool for measuring liquid volume is the graduated cylinder. The volume is measured at the bottom of the meniscus, or curve of the liquid in the cylinder. Common measures of liquid volume are the milliliter (ml) and liter (l). One milliliter is equal to 1 cubic centimeter (cm3). A liter is 0.26 gallons, or a little more than a quart.
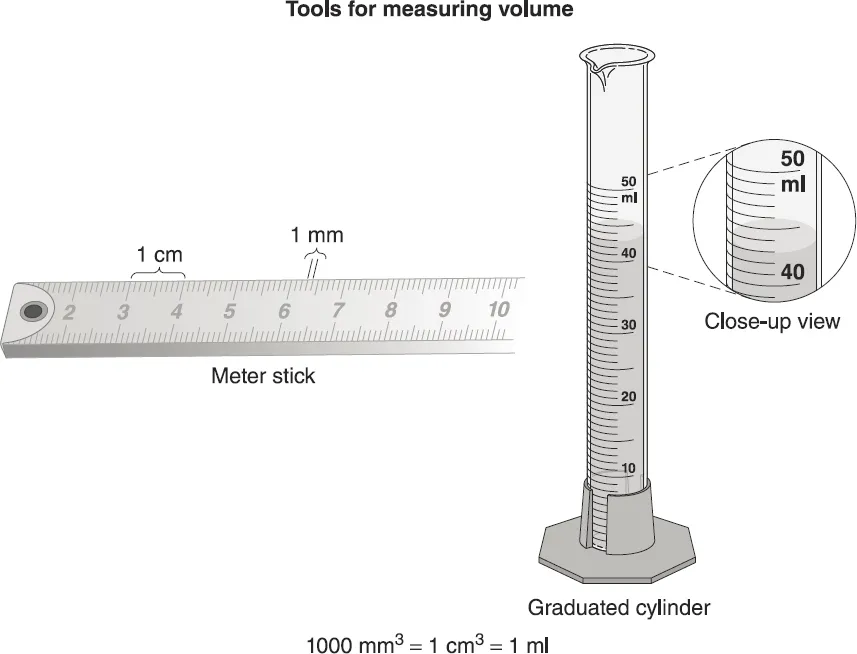
Figure 1–1. Measuring tools
VOLUME
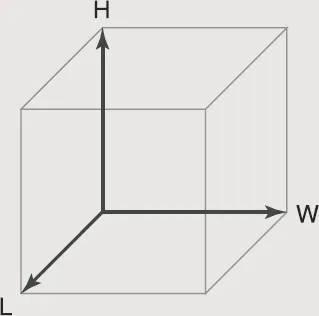
Figure 1–2. Cube
Volume is the space that a substance occupies.
Volume of a Regular Object
The volume of a regular object can be calculated by multiplying the length by the width by the height of...
Table of contents
- Cover
- Title Page
- Dedication
- Copyright
- Contents
- How to Use This Book
- Chapter One: Matter
- Chapter Two: The Periodic Table of the Elements
- Chapter Three: Phases of Matter
- Chapter Four: Gas Laws
- Chapter Five: Mixtures
- Chapter Six: Molecules and Compounds
- Chapter Seven: Chemical Reactions
- Chapter Eight: Acids, Bases, and Salts
- Chapter Nine: Nuclear Reactions
- Chapter Ten: Environmental Chemistry
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Painless Chemistry by Barron's Educational Series,Loris Chen in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.