
This is a test
- 292 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Fimbriae Adhesion, Genetics, Biogenesis, and Vaccines
Book details
Book preview
Table of contents
Citations
About This Book
Fimbriae are the best-studied bacterial colonization factors. They are of paramount importance in bacterial pathogenesis and microbial ecology. Due to the advent of new and powerful techniques, an impressive amount of information has been accumulated on these important surface organelles over the last decade. The first book of its kind, Fimbriae brings together into one volume the state of the art of this very active field. Internationally recognized researchers give both a horizontal and lateral approach to fimbriology. Selected types of fimbriae are extensively reviewed and fundamental questions such as evolution, control or regulation, biogenesis, bacteria-host interaction, and fimbriae-based vaccines are examined.
Frequently asked questions
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes, you can access Fimbriae Adhesion, Genetics, Biogenesis, and Vaccines by Per Klemm in PDF and/or ePUB format, as well as other popular books in Medicine & Pathology. We have over one million books available in our catalogue for you to explore.
Chapter 1
Type 1 Fimbriae of Escherichia coli
Per Klemm and Karen Angeliki Krogfelt
TABLE OF CONTENTS
I. | Introduction | ||
II. | Morphology, Structure, and Composition A. Morphology and Structure B. The Components of Type 1 Fimbriae | ||
III. | Genetics A. The fim Genes B. Regulation of Fimbrial Expression | ||
IV. | Biogenesis A. Fimbrial Subunit Secretion and Processing B. Organelle Assembly | ||
V. | Adhesive Features of Type 1 Fimbriae A. Receptors for Type 1 Fimbriae B. Biological Role of Type 1 Fimbriae, Pathogenesis | ||
VI. | Concluding Remarks | ||
Acknowledgments | |||
References | |||
I. INTRODUCTION
During the last few decades a number of aspects related to the molecular biology of type 1 fimbriae of Escherichia coli have been investigated using a variety of approaches, including classical serology and immunology, molecular genetics, and cell biology.
Type 1 fimbriae are characterized by their ability to mediate agglutination of guinea pig erythrocytes in the absence of α-D-mannose but not in its presence (term: mannose-sensitive agglutination).1 Type 1 fimbriae, often referred to as somatic or common fimbriae, are found on many different species of Enterobacteriaceae. The diversity of fimbrial antigenic types within this family was initially determined by standard bacterial agglutination assays, using whole-cell antisera adsorbed with nonfimbriated variants.2 From these studies, close serologic relationships were found to exist among type 1 fimbriae of Escherichia, Klebsiella, and Shigella spp., whereas the fimbriae of Salmonella and Citrobacter spp. constituted a second serologic group. In addition, the type 1 fimbriae of Enterobacter, Edwardsiella, Hafnia, Serratia, and Providencia spp. each comprised a distinct serologic group.3 DNA probe hybridization has been used to detect homologous DNA sequences in a large number of bacteria representing 11 genera of Enterobacteriaceae. The DNA probe used included the entire structural fimbrial gene and one of the regulatory genes of E. coli. The only bacterial isolates that exhibited sufficient sequence homology to give a signal were the E. coli and Shigella spp. Of 69 E. coli strains tested, only 9 failed to hybridize, thereby confirming the abundance of the type 1 fimbriae among the E. coli.4 The serologic and hybridization results indicated that although type 1 fimbriae are functionally equivalent in the family of Enterobacteriaceae, the genes encoding at least two components have undergone considerable divergence from any common ancestral gene. Furthermore, three antigenically distinct but related type 1 fimbrial variants, with highly homologous N- and C-terminal structures, were found on uropathogenic strains of E. coli. These variants were termed types 1A, 1B, and 1C.5
Type 1 fimbriae have been purified from E. coli,6 Salmonella typhimurium,1 Klebsiella pneumoniae,11 Serratia marcescens,9 and Enterobacter cloacae.10 In all cases, the fimbriae are seen to be polymers consisting mainly of a single protein with a molecular weight of 17 to 21 kDa. The amazingly frequent occurrence of type 1 fimbriae in Enterobacteriaceae and the observation that as many as 80% of all wild-type E. coli strains possess this adhesive organelle, has attracted the attention of many research groups.
Although type 1 fimbriae are widespread in Enterobactericeae, the present review concerns itself with type 1A fimbriae of E. coli as the best characterized and very representative variant.
II. MORPHOLOGY, STRUCTURE, AND COMPOSITION
A. MORPHOLOGY AND STRUCTURE
A typical type 1-fimbriated E. coli K-12 cell contains 100 to 500 fimbriae, each with a width of approximately 7 nm and ranging between 0.2 and 2.0 μm in length. The fimbriae are arranged peritrichously around the cell (Figure 1). The type 1-fimbrial organelle consists mainly of a single structural protein. X-ray crystallo-graphic studies of pseudocrystalline aggregates of type 1 fimbriae indicated a helical structure with 3.125 subunits per revolution and a subunit pitch distance of 2.3 nm with an inner diameter of 2 to 2.5 nm.11 X-ray diffraction studies done by Mitsui gave similar results.12 Subunits are arranged in a right-handed cc-helix, each turn consisting of 3.14 subunits and an axial rise per subunit of 0.8 nm.
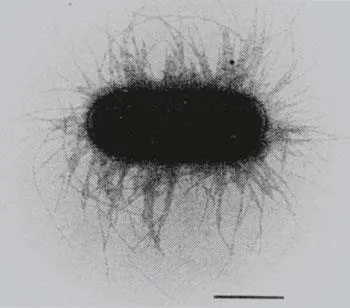
The structural components of the fimbrial organelle are not covalently linked but are held together by hydrophilic and hydrophobic bonds in a very rigid and stable structure. The fimbriae are observed to be highly resistant to proteolysis with trypsin13 and to resist disruption by various denaturing agents, such as SDS and 6M urea, which readily disturb most protein polymers.6 Dissociation of the fimbriae is only possible after incubation at 37° C in saturated guanidine hydrochloride14 or after boiling the fimbriae at low pH, e.g., hydrochloric acid, pH < 1.8.11,15 As soon as the detergents are removed, the fimbrial subunits rapidly repo-lymerize. The rate of this reaction depends upon protein concentration, temperature, pH, salt concentration, and ionic species.11
B. THE COMPONENTS OF TYPE 1 FIMBRIAE
Type 1 fimbriae are heteropolymers. A single organelle consists of about 1000 components of a major structural protein, FimA, and in addition to this contains three other proteins, FimF, FimG, and FimH, which make up 1 to 2% of the total fimbrial protein.16 The first clue as to the presence of other structural proteins than the major structural protein, FimA, came from genetic studies. Analysis of deletion mutants in which the defects were located in the distal part of the fim cluster revealed that this sector contained information vital to the adhesive potential of type 1 fimbriae.17, 18, 19 DNA sequence analysis of the region proved it to contain three genes: fimF, fimG, and fimH.19
Biochemical analysis of fimbriae subsequently revealed that minor components were indeed integral parts of the organelles. Two-dimensional gel electrophoresis of purified fimbriae from recombinant bacteria containing various combinations of the fim genes encoding the structural proteins revealed that wild-type fimbriae contain four proteins, i.e., the major structural protein, FimA with an apparent molecular weight of 17.0 kDa, and three proteins present as minor components with molecular weights of 17.0, 18.0, and 30.0 kDa, respectively. These observations corresponded to the molecular weights deduced from the DNA sequence data. Furthermore, a clone that did not contain the genes encoding the minor component proteins did produce fimbriae consisting of pure FimA protein, indicating that the minor components were not necessary for the structural integrity of the fimbriae; however, such organelles were nonadhesive and did not confer hemagglutination. Moreover, pure FimA fimbriae were observed to be very rigid structures that could only be depolymerized under extreme conditions.16 Another study, employing a different approach based on immunological detection of minor components with antibodies raised against synthetic peptides corresponding to the N-terminal parts of the FimG and FimH proteins, confirmed that these are integral parts of the fimbriae.20 Immunoelectron microscopy revealed that the FimH protein was present at the fimbrial tips and at long intervals along the fimbrial organelles.
Several studies using polyclonal antibodies against the minor components to prevent hemagglutination suggested that FimH is the adhesive molecule of type 1 fimbriae.21,22 By an elegant method directly relying on the lectin nature of the adhesin, i.e., binding to mannose moieties, it was shown that the molecule uniquely responsible for the adhesive activity of type 1 fimbriae is indeed the FimH protein. Furthermore, it was shown that the adhesin is positioned integrated in and along the fimbrial rod.23
Fimbriae consisting of all the structural components, i.e., FimA, FimF, FimG, and FimH, were observed to be much more prone to breakage and depolymerization than pure FimA fimbriae. This suggests that the minor components introduce fragile points at intervals along the fimbriae.16 In the case of broken fimbriae, the adhesin would be exposed to the tip of the fimbriae. Furthermore, several lines of evidence indicate that the minor components FimF and FimG are required for integration of the FimH adhesin into the organelle at a tip location but also at intervals along the fimbrial shaft. Based on the above results, a reasonable understanding of the architecture of the type 1-fimbrial organelle has been reached (Figure 2).
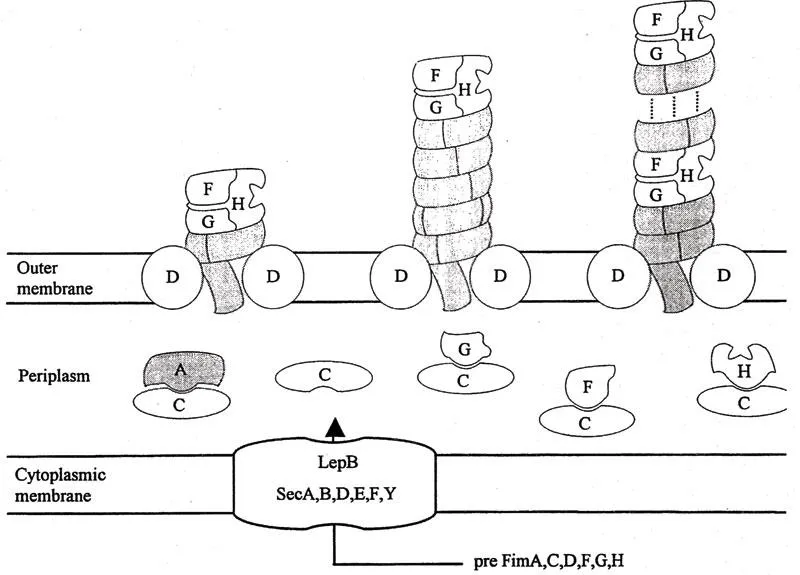
Table of contents
- Cover
- Title Page
- Copyright Page
- Preface
- The Editor
- Contributors
- Table of Contents
- Introduction: A Historical Perspective
- Chapter 1 Type 1 Fimbriae of Escherichia coli
- Chapter 2 S and F1C Fimbriae
- Chapter 3 Structure, Function, and Biogenesis of Escherichia coli P Pili
- Chapter 4 Fimbriae of Enterotoxigenic Escherichia coli
- Chapter 5 Nonfimbrial Adhesins of Escherichia coli
- Chapter 6 Type 3 Fimbriae of the Enterobacteriaceae
- Chapter 7 Salmonella Fimbriae
- Chapter 8 Bordetella pertussis Fimbriae
- Chapter 9 Type 4 Fimbriae
- Chapter 10 Pili (Fimbriae) of Neisseria gonorrhoeae
- Chapter 11 Fimbriae of Vibrio cholerae
- Chapter 12 Regulation of E. coli Fimbrial Expression
- Chapter 13 Fimbrial Operons and Evolution
- Chapter 14 Bacteria-Extracellular Matrix Interactions
- Chapter 15 The Role of Interactions Between Fimbriae and the Intestinal Mucus Layer in Bacterial Colonization and Enteric Pathogenesis
- Chapter 16 An Ecological Perspective of Fimbrial Function in the Porcine Digestive Tract
- Chapter 17 Fimbriae and Disease
- Chapter 18 Fimbrial Vaccines
- Chapter 19 Chimeric Fimbrial Vaccines
- Index