![]()
1
Introduction to Carbon and Carbon Nanomaterials
1.1 Introduction
Carbon is the fifteenth most abundant element in the earth’s crust, and the fourth most abundant element in the universe by mass after hydrogen, helium, and oxygen. Carbon is also present as a major component in large masses of carbonate rocks, including limestone, dolomite, and marble. Coal is the largest commercial source of mineral carbon, accounting for nearly 8 billion tons or almost 80% of fossil carbon fuel. In its elemental form, carbon (C, atomic number 6) has a valency of 4 and is therefore placed in group IV of the periodic table along with Si, Ge, Sn, and Pb.
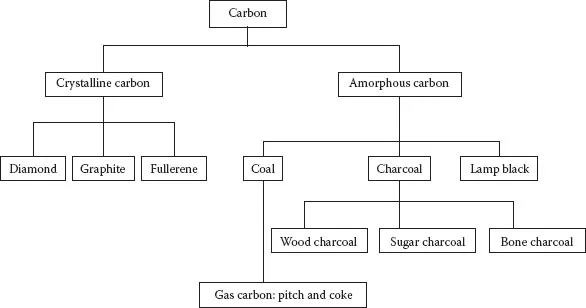
Carbon can exist in both crystalline and amorphous forms. Figure 1.1 shows the three crystalline allotropes of carbon, that is, with same chemical properties but with different physical forms. All carbon allotropes are solids under normal conditions, with graphite being the most thermodynamically stable form. In reality all the allotropes of amorphous carbons are made of microcrystals of graphite arranged in an irregular fashion. Diamond, graphite, and fullerenes are crystalline allotropes of carbon. The carbon atoms in diamond have a three-dimensional (3D) tetrahedral network of covalent bonds, which causes the electrons to be held tightly. Diamonds are therefore very hard and have high melting and boiling points. The structure is a closely packed structure and causes diamond to be denser than graphite. Since all its electrons end up in forming the covalent bonds, therefore it does not conduct electricity. In graphite only three of the four valence electrons of each carbon atom are used in bonding, leaving the fourth valence electron as free. Therefore graphite is a good conductor of electricity.
Diamond is transparent because it has no free electron to absorb radiations and make a transition in the optical region. Graphite on the other hand has one free electron, which can absorb all the radiations in the optical region and thus appear black. Figure 1.2 shows the two contrasting physical forms of naturally occurring allotropes of carbon, which are used for many industrial applications.
Recently fullerenes have been discovered as the third allotrope of carbon. They are molecular compounds made entirely of carbon atoms in the form of hollow spheres (C60), ellipticals (C70), and tubes. Spherical fullerenes are sometimes called buckyballs, and cylindrical fullerenes are called buckytubes or nanotubes. The fullerenes might have applications in synthetic cosmetics, pharmaceuticals, and organic photovoltaics.
Carbon that is deposited on the walls of the reactor during the destructive distillation of coal is called gas carbon and results into coal tar pitch. Carbon that is deposited on the walls of the distillation tower during the refining of crude petroleum is called petroleum coke. Both forms are used for making electrode in dry cells and are good conductors of electricity.
FIGURE 1.1
Various physical forms of carbon.
FIGURE 1.2
Physical forms of graphite and diamond crystals.
Wood charcoal is made by the destructive distillation of wood. It is a black, porous, brittle solid that is a good adsorbent, and good filtering and reducing agent.
Sugar charcoal is obtained by heating sugar in the absence of air. It is the purest form of amorphous carbon. It can be converted into activated charcoal to increase its adsorption capacity.
Animal charcoal is also called bone char. It is obtained by destructive distillation of bones. It contains about 10% to 12% of amorphous carbon.
Carbons have always been the friendliest materials to mankind. Charcoal from wood was the primitive artist’s crude element for expression and communication as mural on cave walls. A candle burning with yellow flame would produce soot on an inverted plate and the black deposit known as soot (lamp black) when mixed with suitable base (water, oil) created pigments.
The advent of 20th century saw widespread use of graphite crystals as the writing points for several centuries and has now taken the form of lead in pencils. Printing formed the first major use of carbon in manufacturing technologies, many of which are still in use today.
Starting in 1940, controlling the processing parameters together with the variety of choices from carbonaceous materials, individually or in combination led to the development of advanced carbon products with contrasting properties. Examples include carbons that possess the highest thermal conductivity (carbon nanotubes, graphene, carbon–carbon composites), but at the same time are thermal insulators (carbon fiber felt/porous carbons), completely impervious to gases and fluids (glassy carbon with no open porosity) to highly porous carbons (activated carbon, surface area ~2000 m2/g), very soft (graphite) and hardest (diamond), very low coefficient of friction (natural graphite, expanded graphite) to highly abrasive (hard carbons), low strength (monoliths) to strongest material (carbon fibers, carbon nanotubes, graphene). Because of such versatility, today carbon has entered into applications ranging from conventional carbon/graphite electrodes, brushes, clutch plates, mechanical seals and catalyst support, to highly advanced areas like carbon–carbon composites for space re-entry vehicles, aircraft brake discs, and carbon fiber composites for lightweight, high-strength structures.
The chapter is aimed to provide to the reader a brief idea about the basic structure of carbon material in the bulk form and how the discovery of the nanostructured forms of carbon has opened up use of carbon in newer applications. Since the majority of carbon materials are a result of sp2 hybridization, the structure is explained on the basis of natural or single-crystal of graphite. This will form the basis of the understanding of electronic and band structure of nanoforms of carbon presented in the following chapters. X-ray diffraction and Raman spectroscopy are two important characterization tools that can give a lot of insight into the structure of various forms of carbon and are widely used by researchers to understand the novel and newer forms of nanocarbon. An introductory reference is also provided to x-ray diffraction and Raman spectroscopy used to characterize the structure of various forms of carbon and should help the reader in understanding the difference when we move from bulk to carbon nanomaterials.
1.1.1 Atomic Structure of Carbon
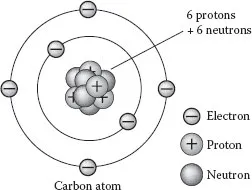
Carbon is a chemical element with the symbol C and atomic number 6 and represented by the electronic orbital structure illustrated in Figure 1.3. As a member of group IV of the periodic table, it is obtained in three naturally occurring isotopes, with 12C (6 protons, 6 neutrons, nuclear spin I = 0) and 13C (6 protons and 7 neutrons, nuclear spin I = 1/2) being stable, whereas 14C (6 protons and 8 neutrons) is radioactive, decaying with a half-life of about 5700 years. The carbon-12 (12C) isotope forms almost 99% of the carbon on earth, while carbon-13 (13C) forms the remaining 1%. Though 14C is only found in traces (~10−12 of all carbon atoms), due to its long half-life corresponding to reasonable life scale in human history, measurement on 14C concentration in organic material (e.g., wood) allows one to date its biological history; this is called carbon dating.
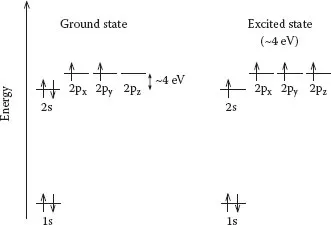
A carbon atom with six electrons has the electron configuration in its ground state (lowest energy state), as shown in Figure 1.4. Note that there is a large energy difference between the 1s and 2s orbital and a very small difference in energy between 2s and 2p orbitals. The 1s2 orbital contains two strongly bound core electrons. Four more weakly bound electrons occupy the 2s22p2 valence orbitals. In the crystalline phase, the valence electrons give rise to 2s, 2px, 2py, and 2pz orbitals, which are important in forming covalent bonds within carbon materials. Because the energy difference between the upper 2p energy levels and the lower 2s in carbon is small (~4 eV) compared to the binding energy of the chemical bonds, the electronic wave functions for these four electrons can readily mix with one another, thereby changing the occupation of the 2s and three 2p atomic orbitals, so as to enhance the binding energy of a C atom with its neighboring atoms. The general mixing of 2s and 2p atomic orbitals is called hybridization, whereas the mixing of a single 2s electron with one, two, or three 2p electrons is called spn hybridization with n = 1, 2, 3.
FIGURE 1.3
Orbital representation of carbon atom.
FIGURE 1.4
Electronic configuration for carbon in the ground state and in the excited state.
The vertical arrows in Figur...