Water present below the land surface takes on some of the characteristics of that environment. Rainfall and snowmelt percolating through the soil zone and unsaturated material chemically react with the gases, minerals, and organic compounds that occur naturally in the subsurface. These reactions continue below the water table as the water flows through the aquifer. The result is that the characteristics and composition of the water evolve as it flows through the ground in response to the types of solid and gas phases that the solution encounters and the geochemical reactions that occur between these phases. Part I of this book describes the fundamentals of groundwater geochemistry. Chapter 1 discusses characterization of the water/rock system, and Chapters 2 and 3 discuss solution/gas and water/rock reaction processes. The development of geochemical models to simulate the natural system is described in Chapter 4, while Chapter 5 provides a discussion of the computer codes commonly used to model groundwater geochemical reactions.
1 The Groundwater Geochemical System
Groundwater supplies drinking water to over half the population of the United States and provides an even greater percentage of the amount of water used for irrigation and industrial purposes.1 Thirty years ago the main use of geochemistry to groundwater was as an aid in differentiating water types in aquifers to help identify and quantify water resources. Today, geochemistry plays a much larger role in groundwater studies because of its importance in characterizing the natural system, understanding contaminant migration, and designing remediation programs. Neglecting the geochemistry component of groundwater contamination may tremendously oversimplify the situation and lead to erroneous conclusions regarding the impact of contamination on the environment and the amount of effort required to remediate the aquifer.
The area of geochemistry that we are interested in is low temperature, aqueous geochemistry that focuses on water/rock interactions in the unsaturated zone and below the water table in the aquifer(s) present at a site. These water/rock interactions are the geochemical processes that soil scientists have studied for decades to optimize plant growth and that groundwater scientists/engineers are now using to better understand the chemical facet of subsurface conditions. Along with the physical and biological processes active in the subsurface, geochemistry plays a major role in controlling groundwater composition and the movement of dissolved constituents.
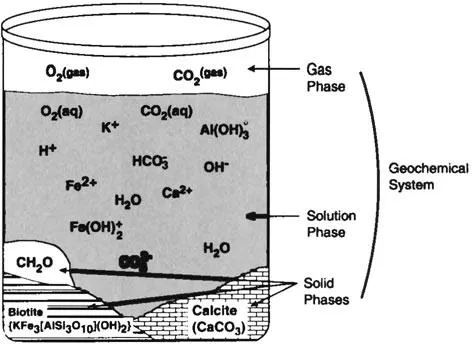
The subsurface can be thought of as a dynamic geochemical system consisting of (1) solid phases (minerals, amorphous [noncrystalline] solids, and organic matter), (2) a soil gas phase, and (3) an aqueous solution phase (water with its dissolved constituents). Each phase is a separate physical entity with more or less uniform chemical composition. There is commonly a single gas phase and a single solution phase, although two or more separate solution phases (such as surface and groundwater) may mix to produce a new solution phase. Each mineral type or other solid material with the same composition and properties is considered a separate phase. A system is simply an assemblage of these solid, gas, and solution phases under site-specific temperature and pressure conditions. The beaker model shown in Figure 1-1 represents a simple geochemical system. The five phases comprising this system are solution, gas, organic matter (CH2O), biotite, and calcite. Water is in contact with the gas phase and the various inorganic/organic solid phases. Some of the constituents of the solution phase may have been present as dissolved in the water when it was added to the beaker, whereas others may have dissolved into the water as a result of reactions in the beaker between the phases.
In the natural geochemical system, fresh recharge water with few dissolved constituents and low concentrations contacts the subsurface material and interacts with the other phases of the system. Chemical reactions occur because the composition of the recharge water is not in equilibrium with the solid phases or the soil gases. Disequilibrium drives the reactions that dissolve gases an...