![]()
© IWA Publishing 2020. Physical and Chemical Separation in Water and Wastewater Treatment Author(s): Norihito Tambo and Koichi Ogasawara doi: 10.2166/9781789061307_0001
Chapter 1
Earth: The water planet
1.1 THE PRESENCE OF WATER ON EARTH
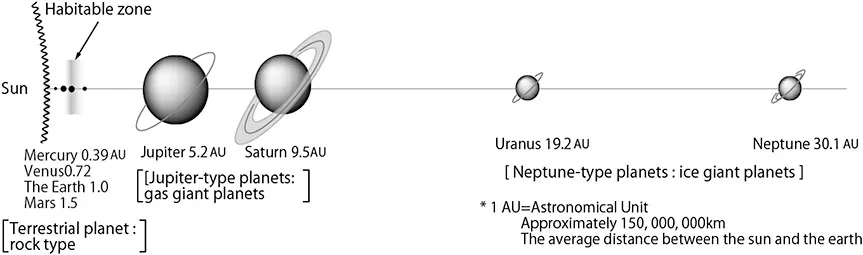
The Earth is called the ‘water planet’. It sits in the only planetary system (the solar system, centered on the sun), in which water can exist in large quantities in liquid form. Situated in the so-called ‘habitable zone’, the Earth is 149.6 million kilometers from the sun (this distance being known as one astronomical unit, or 1AU), and has a mass which is adequate enough to keep water and other gases around it by gravity. Figure 1.1 shows the relative distances from the sun and magnitudes of all the solar planets.
Figure 1.1The Earth and the solar system.
1.1.1 Planets of the solar system
Mercury is a rocky, terrestrial planet and is the closest planet to the sun with an average distance of 0.387AU: about 0.580 billion km. Its surface temperature ranges from −170 to +430°C. On Mercury, water occurs as a gaseous molecule, due to the largely high temperature environment which exists there. Because of the small mass of the planet (0.055 times that of the earth), its gravity is small, so water molecules are not able to be held in the atmosphere and they dissipate into outer space.
The Moon has an even smaller mass so that it cannot hold water and other gases around it, despite being in the same habitable zone as the Earth.
Mercury, Venus (average distance from the Sun: 0.7233AU; average surface temperature +480 C; mass ratio to the Earth 0.81), the Earth (1.00AU; average surface temperature +17°C) and Mars (1.5237AU; average surface temperature −45°C; mass ratio 0.11) are referred to as the inner planets inside the asteroid belt. There is a tiny probability of water on rock-type (Earth-type) planets. However, the temperature is too high on Venus and it is too cold on Mars for water to exist as a liquid. Mars seems to have water in the form of ice on the ground but water molecules in the atmosphere are thought to have been gradually lost because of the small gravity force of the planet.
Jupiter (5.2025AU; −120°C), Saturn (9.5549AU; −180°C), Uranus (19.2148AU; −210°C) and Neptune (30.1104AU; −220°C) are called the outer planets. Although the density of these planets is 0.13 to 0.30 times smaller than that of the Earth, their mass is larger, being 318 times larger than that of the Earth (in the case of Jupiter) and 17.1 times larger than that of the Earth (in the case of Neptune), and they are thought to exist as high-density low-temperature gaseous bodies. Jupiter and Saturn are classified as Jupiter-type planets (gas giant planets), and Uranus and Neptune are classified as Neptune-type planets (ice giant planets). These planets are thought to have a core in which the body of gas freezes but do not have a solid surface like the Earth, instead being covered with a body of gas.
1.1.2 Earth, the water planet
Among the planets of the solar system, only the Earth continues to hold a large amount of liquid water on its surface; because of the distance from the sun (1AU), the surface temperature remains at an average +17C and water can therefore exist as a liquid. At the same time, the gravitational acceleration (G = 1) due to the Earth's mass (6 × 1024 kilograms, which is about nine times larger than that of Mars) has enough force to restrain gas molecules, and water molecules that have become water vapor are also stopped from flying out into space.
Another factor that constitutes a condition for water to exist as a liquid at normal temperatures on the Earth is the so-called greenhouse gases including atmospheric water vapor, carbon dioxide, methane, and nitrogen dioxide. This greenhouse gas ‘jacket’ wrapped around the earth stores some of the heat radiated into outer space on the earth's surface. Without these gases, the atmosphere would become −8°C, resulting in a frozen Earth. The greenhouse effect of these gases has raised the surface temperature to a normal temperature of +17°C. If the concentration of greenhouse gases becomes too high, it could produce a very hot atmosphere with a surface temperature as high as +480°C, like Mercury. The Earth became a water planet under the three subtle conditions described above, and became a habitable planet, a planet for the well-being of living things, by distributing solar energy to various processes on Earth using water as a medium. Thus, while physics and chemistry are universal basic sciences which lead us to space, biology is limited as a science that only works on a habitable planet. In search of the traces and existence of living things in outer space, biological science is now being studied in an attempt to achieve the same universality as physics and chemistry (Figure 1.2).
Figure 1.2The conditions under which water can exist in liquid form.
1.1.3 Origin of the Earth's water
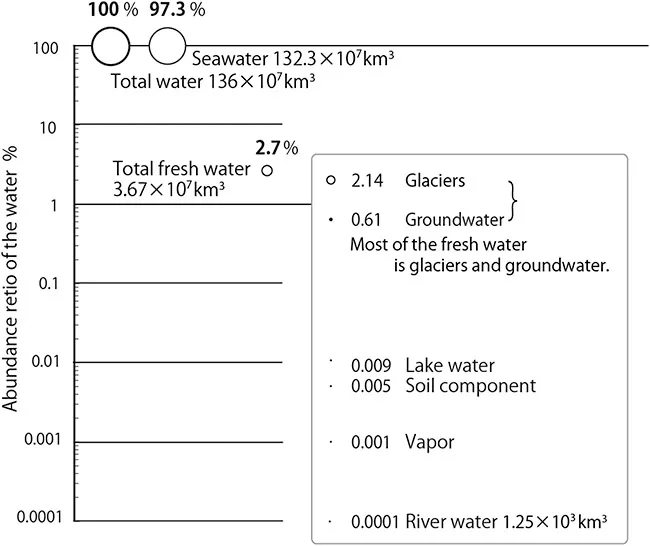
How does liquid water, which characterizes the Earth as a habitable planet, appear and exist on Earth? Although the Earth is said to be a water planet, the mass of the ocean (1.4 × 1021 kg) is only 0.023% of the total mass (weight) of the Earth (6.0 × 1024 kg). Table 1.1 shows the percentage of water on the planet. Seawater accounts for 97.3% of the total water mass. Fresh water accounts for only 2.7% of the total, most of which is confined in glaciers in the Polar Regions, whilst only 0.01 to 0.02% of total water resources used in ecosystems and human social systems are river water, lake water and shallow groundwater. A graph of Table 1.1 appears in Figure 1.3.
Figure 1.3The presence ratio of the Earth's water.
Table 1.1 The water mass of the Earth.
| Existence State of the Water | Each Mass | Presence Ratio |
| Total mass | 136 × 107 km3 | 100% |
| Sea water | 132.3 × 107 km3 | 97.3% |
| Fresh water | 3.67 × 107 km3 | 2.7% |
| Vapor (in the atmosphere) | 13 × 103 km3 | 0.001% |
| River water | 1.25 × 103 km3 | 0.0001% |
| Lake water | 125 × 103 km3 | 0.009% |
| Groundwater | 835 × 104 km3 | 0.61% |
| Soil component | 67 × 103 km3 | 0.005% |
| Glaciers in Polar Regions | 2.92 × 107 km3 | 2.14% |
A variety of studies are underway on the origins of how the Earth's water was acquired. Broadly speaking, it is thought that water was generated and accumulated in the Earth's formation process by collision coalescence of planetesimals, in which water-containing minerals in the form of hydroxyl groups and ice became materials. The origin of the overwhelmingly large percentage of sea water are derived from chondrite meteorites from when the Earth was formed by planetesimal collisions 4.6 billion years ago, or from H2O which is the main component of comets, and from primitive circumstellar disks of gases in the process of forming the dynamics of the solar system.
1.1.4 Water circulation
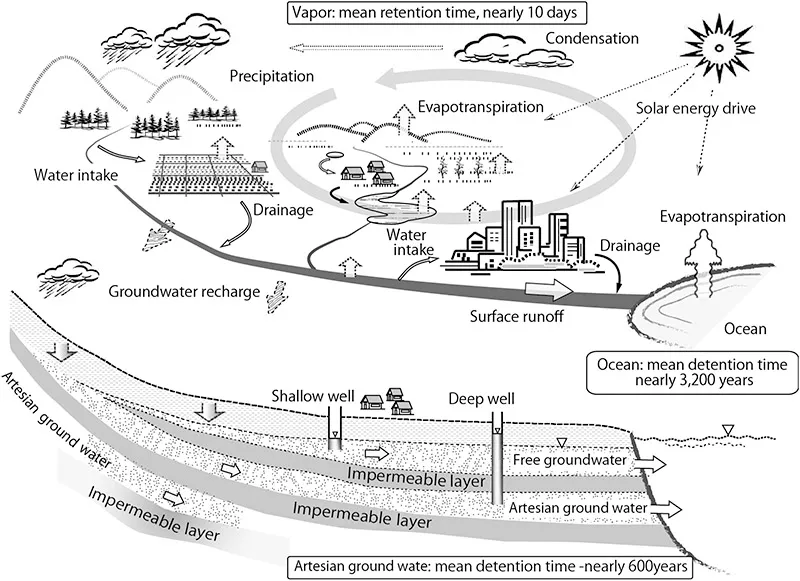
The fresh water on the Earth is constantly recycled by solar energy to maintain the life and environment of the planet. Figure 1.4 schematically illustrates the hydrological cycle that forms the basis for the maintenance of humans and ecosystems. Water vapor returns to the ground as precipitation in a short period of 10 days of mean retention time in the sky, and it then becomes surface water/groundwater to be used to grow organisms.
Figure 1.4The hydrological cycle.
Figure 1.5 shows the total global water fluxes produced by Spiedel and Agnew (1982). The ocean has an overwhelmingly large storage volume that forms the basis for water circulation. The ocean has a mean depth of 3500 m, and has a retention time of nearly 3200 years in relation to evaporative volume. When co...